Alkalosis
Alkalosis refers to a condition reducing hydrogen ion concentration of arterial blood plasma (alkalemia). Generally alkalosis is said to occur when pH of the blood exceeds 7.45. The opposite condition is acidosis.
Types
More specifically, alkalosis can refer to:
• Respiratory alkalosis
• Metabolic alkalosis
Causes
The main cause of respiratory alkalosis is hyperventilation, resulting in a loss of carbon dioxide. Compensatory mechanisms for this would include increased dissociation of the carbonic acid buffering intermediate into hydrogen ions, and the related consumption of bicarbonate,[citation needed] both of which would lower blood pH.
Metabolic alkalosis can be caused by prolonged vomiting, resulting in a loss of hydrochloric acid with the stomach content. Severe dehydration, and the consumption of alkali are other causes. It can also be caused by administration of diuretics and endocrine disorders such as Cushing's syndrome. Compensatory mechanism for metabolic alkalosis involve slowed breathing by the lungs to increase serum carbon dioxide, a condition leaning toward respiratory acidosis. As respiratory acidosis often accompanies the compensation for metabolic alkalosis, and vice versa, a delicate balance is created between these two conditions.
Complications
Metabolic alkalosis is usually accompanied with hypokalemia, causing e.g. muscular weakness, myalgia, and muscle cramps (owing to disturbed function of the skeletal muscles), and constipation (from disturbed function of smooth muscles).
It may also cause hypocalcemia. As the pH of blood increases, the protein in the blood becomes more ionised into anions. This causes the free calcium present in blood to bind strongly with protein. If severe, it may cause tetany (alkalotic tetany).
الثلاثاء، 19 مايو 2009
الأربعاء، 1 أبريل 2009
Graves Disease
Overview of Graves' Disease
Graves’ disease as it is known in the United States and von Basedow’s disease or Parry’s disease in other countries belongs to the organ-specific autoimmune diseases category and is an example of a type II hypersensitivity reaction (McGregor et al., 1998). Graves’ disease is classified by the production of auto-antibodies that bind to the thyroid-stimulating hormone (TSHR) receptor, which is located on the thyroid gland.This action causes an overproduction of thyroid hormones, which is called hyperthyroidism. Graves’ disease is the most common autoimmune disorder that causes hyperthyroidism (Wikipedia contributors et al., 2006).
In Graves’ disease the feedback regulation of thyroid hormone production is disrupted. Normally, the pituitary gland secretes thyroid-stimulating hormones (TSH), which acts on the thyroid inducing the release of thyroid hormones. Feedback suppression occurs when the thyroid hormones act on the pituitary gland suppressing further development of thyroid hormones. Autoimmune B cells that are activated due to autoimmunity secrete auto-antibodies that bind to the TSH receptor located on the thyroid follicle, which also induces thyroid hormone production. The thyroid hormone continues to do its normal job as well as suppressing further development of thyroid hormones by acting on the pituitary gland but has no effect on autoantibody production, which continues to stimulate extreme thyroid hormone production
Figure 1. Is an illustration of excess thyroid hormone production by the thyroid follicle due to auto-antibody production (Janeway et al., 2005).
--------------------------------------------------------------------------------
Symptoms of Graves' Disease
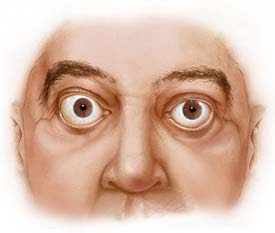
The most common symptoms of Graves’ disease include; diffuse goiter (enlarged thyroid gland), weight loss, rapid heart beat, fatigue, muscle weakness, frequent bowel activity, restlessness, heat sensitivity, hand tremors and increased sweating (National Women's Health Information Center et al., 2006). In addition, certain individuals with Graves' disease display symptoms associated to this form of hyperthyroidism, including infiltrative ophthalmopathy (Figure 2. bulging eyes) and pretibial myxedema (swelling of shins), which occur at lower extremes
Figure 2. This is what a patient's eyes would like if they were infected with Graves' opthalmopathy. Figure taken pending permission from http://www.mayoclinic.org/graves-disease/symptoms.html
--------------------------------------------------------------------------------
Risk Factors
Susceptibility to Graves' disease can be determined by several factors, although the process of initiation is still a mystery. Graves’ disease can occur in genetically susceptible individuals when an environmental factor, possibly an infectious agent damages thyroid tissue leading to the exposure of self-antigens to the immune system. It also has been determined that throughout Graves’ disease appearance it is most common in middle-aged women. There is an 8:1 ratio compared to men. However, it is not scarce in adolescent years, the period of pregnancy or in people over the age of fifty (National Women's Health Information Center et al., 2006).
Different factors of evidence have proposed that Graves' disease occurs in families. Horwitz and Refetoff et al. (1977) found and proprosed that Graves' disease has an increased occurrence in patients with familial deficiency of thyroxine-binding globulin.
Unfortunately, Graves’ disease can be passed through pregnancy. Mothers with this disease transfer their auto-antibodies which are IgG mediated across the placenta into the fetus. The newborn develops slight symptoms of Graves’ disease. Therefore, newborns are said to be born with hyperthyroidism. This transfer of antibody-mediated autoimmune disease can be fixed by a process called plasmapheresis, which replaces the newborn’s plasma with normal plasma, thus eliminating the transferred maternal antibody (Janeway et al., 2005).
Organ-specific autoimmune diseases are considered to occur frequently together in many combinations; for example, autoimmune thyroid disease and vitiligo (autoimmune depigmenting disease) are occasionally found in the same individual. In addition, these diseases can also be recognized with associations of Human Leukocyte Antigen (HLA) serotype. (Janeway et al., 2005). Association of HLA serotype with susceptibility to Graves’ disease is with the HLA-DQ gene, which is in linkage to the DR genes. The HLA allele associated with Graves’ disease is said to be allele DR3 (McGregor et al., 1998). We do not want to overlook genetic factors but they are not determinative for predisposition to Graves' disease.
Genetic analysis has shown that Graves’ disease is associated with the polymorphisms in the cytotoxic T-lymphocyte antigen 4 (CTLA-4) genes (DeGroot et al., 2005). Allele sequence of A/G or G/G on nucleotide 49 of the CTLA-4 gene in patients have shown that more people are infected with Graves' Disease (Kouki et al., 2000).
--------------------------------------------------------------------------------
Immune System and Graves' Disease
The thyroid gland is vital in maintaining the body’s homeostasis through the secretion of the thyroid hormones, thyroxine (T4) and triiodothyronine (T3). These thyroid hormones are secreted by thyrocytes through fluid phase uptake and lysosomal degradation of the prohormone thyroglobulin (Tg). Hyperthyroidism occurs when there is an overproduction of these thyroid hormones.
The body has a negative-feedback pathway that controls hyperthyroidism (refer back to Figure 1). Immune system signaling is important for hormone synthesis to be controlled. Consider immune system signaling in assisting TSH's up-regulation of hormone synthesis and in turn TSH's down-regulation by thyroid hormones themselves. This cycle is what helps stablize hyperthyroidism from occurring in less susceptible individuals (Lisi et al., 2003).
Graves’ disease can occur in genetically susceptible individuals when an environmental factor, possibly an infectious agent damages the thyroid leading to the exposure of self-antigens to the immune system. Infectious agents have been considered an important environmental inducer of autoimmune diseases. For example, Yersinia Enterocolitica is a common intestinal parasite, which may induce reactivity to TSH receptor. Within the pathogenic bacteria, the TSH receptor could actually appear to be coded for by the bacteria’s plasmids. This concept can be referred as molecular mimicry. A substantial amount of individuals who are affected by Graves’ disease have been infected with this bacteria compared to people who do not have Graves' disease. If this bacterium is exposed to your body as well as your immune system, it can induce TSH receptor antibodies and TSH would appear to bind to a molecule on the bacteria leading to an overproduction of thyroid-stimulating hormones (DeGroot et al., 2005).
Certain cells such as lymphocytes and thyroid can further increase autoimmune responses in Graves' disease. Thyroid cells are able to interact with the immune system causing a response where the symptoms of hyperthyroidism worsen. Unfortunately, thyrocytes that make up the thyroid follicle express and synthesize certain proteins that they normally would not (Weetman et al., 2000). When there is an over-production of thyroid hormones thyroid-reactive T cells are formed and they infiltrate the thyroid gland. In Graves' disease, the majority of the T cells differentiate into Th2 instead of Th1 cells where they can activate B cells which produce TSHR antibodies. This differentiation into Th2 cells allows the stimulation of the thyroid, which causes clinical hyperthyroidism (Davies et al., 2000). In contrast, Hashimoto thyroiditis occurs in the opposite direction as Graves' disease by infilrating T cells differentiating into Th1 cells, which thyroid follicular cells undergo apoptosis leading to clinical hypothyroidism (underactivity/failure of the thyroid) (Weetman et al., 1996).
--------------------------------------------------------------------------------
Current Treatments of Graves' Disease
People who develop this autoimmune disease try to treat it with one of the three different type of treatments such as anti-thyroid drugs, radioactive iodine or surgical thyroid removal. Radioactive idoine is the most common treatment for Graves' disease in North America.
Anti-thyroid drugs can decrease the production of excessive thyroid hormones produced by the thyroid, sustaining thyroid hormone synthesis back to its threshold. Radioactive iodine decreases thyroid hormone levels by damaging thyroid cells, which eventually shrinks the cells leading to their destruction and as well as the functioning of the thyroid gland. Surgical treatment is the removal of the entire thyroid gland, which in many cases people develop an under-active thyroid. If an individual decides to take the surgical route they will have to take thyroid replacement hormones for the duration of their life (National Women's Health Information Center et al., 2006).
The main anti-thyroid drug used in the United States is called methimazole (Wikipedia contributors et al., 2006). “Methimazole…restores euthyroidism by inhibiting thyroperoxidase activity and hormone formation within Tg" (Lisi et al., 2003).
Graves’ disease as it is known in the United States and von Basedow’s disease or Parry’s disease in other countries belongs to the organ-specific autoimmune diseases category and is an example of a type II hypersensitivity reaction (McGregor et al., 1998). Graves’ disease is classified by the production of auto-antibodies that bind to the thyroid-stimulating hormone (TSHR) receptor, which is located on the thyroid gland.This action causes an overproduction of thyroid hormones, which is called hyperthyroidism. Graves’ disease is the most common autoimmune disorder that causes hyperthyroidism (Wikipedia contributors et al., 2006).
In Graves’ disease the feedback regulation of thyroid hormone production is disrupted. Normally, the pituitary gland secretes thyroid-stimulating hormones (TSH), which acts on the thyroid inducing the release of thyroid hormones. Feedback suppression occurs when the thyroid hormones act on the pituitary gland suppressing further development of thyroid hormones. Autoimmune B cells that are activated due to autoimmunity secrete auto-antibodies that bind to the TSH receptor located on the thyroid follicle, which also induces thyroid hormone production. The thyroid hormone continues to do its normal job as well as suppressing further development of thyroid hormones by acting on the pituitary gland but has no effect on autoantibody production, which continues to stimulate extreme thyroid hormone production
Figure 1. Is an illustration of excess thyroid hormone production by the thyroid follicle due to auto-antibody production (Janeway et al., 2005).
--------------------------------------------------------------------------------
Symptoms of Graves' Disease
The most common symptoms of Graves’ disease include; diffuse goiter (enlarged thyroid gland), weight loss, rapid heart beat, fatigue, muscle weakness, frequent bowel activity, restlessness, heat sensitivity, hand tremors and increased sweating (National Women's Health Information Center et al., 2006). In addition, certain individuals with Graves' disease display symptoms associated to this form of hyperthyroidism, including infiltrative ophthalmopathy (Figure 2. bulging eyes) and pretibial myxedema (swelling of shins), which occur at lower extremes
Figure 2. This is what a patient's eyes would like if they were infected with Graves' opthalmopathy. Figure taken pending permission from http://www.mayoclinic.org/graves-disease/symptoms.html
--------------------------------------------------------------------------------
Risk Factors
Susceptibility to Graves' disease can be determined by several factors, although the process of initiation is still a mystery. Graves’ disease can occur in genetically susceptible individuals when an environmental factor, possibly an infectious agent damages thyroid tissue leading to the exposure of self-antigens to the immune system. It also has been determined that throughout Graves’ disease appearance it is most common in middle-aged women. There is an 8:1 ratio compared to men. However, it is not scarce in adolescent years, the period of pregnancy or in people over the age of fifty (National Women's Health Information Center et al., 2006).
Different factors of evidence have proposed that Graves' disease occurs in families. Horwitz and Refetoff et al. (1977) found and proprosed that Graves' disease has an increased occurrence in patients with familial deficiency of thyroxine-binding globulin.
Unfortunately, Graves’ disease can be passed through pregnancy. Mothers with this disease transfer their auto-antibodies which are IgG mediated across the placenta into the fetus. The newborn develops slight symptoms of Graves’ disease. Therefore, newborns are said to be born with hyperthyroidism. This transfer of antibody-mediated autoimmune disease can be fixed by a process called plasmapheresis, which replaces the newborn’s plasma with normal plasma, thus eliminating the transferred maternal antibody (Janeway et al., 2005).
Organ-specific autoimmune diseases are considered to occur frequently together in many combinations; for example, autoimmune thyroid disease and vitiligo (autoimmune depigmenting disease) are occasionally found in the same individual. In addition, these diseases can also be recognized with associations of Human Leukocyte Antigen (HLA) serotype. (Janeway et al., 2005). Association of HLA serotype with susceptibility to Graves’ disease is with the HLA-DQ gene, which is in linkage to the DR genes. The HLA allele associated with Graves’ disease is said to be allele DR3 (McGregor et al., 1998). We do not want to overlook genetic factors but they are not determinative for predisposition to Graves' disease.
Genetic analysis has shown that Graves’ disease is associated with the polymorphisms in the cytotoxic T-lymphocyte antigen 4 (CTLA-4) genes (DeGroot et al., 2005). Allele sequence of A/G or G/G on nucleotide 49 of the CTLA-4 gene in patients have shown that more people are infected with Graves' Disease (Kouki et al., 2000).
--------------------------------------------------------------------------------
Immune System and Graves' Disease
The thyroid gland is vital in maintaining the body’s homeostasis through the secretion of the thyroid hormones, thyroxine (T4) and triiodothyronine (T3). These thyroid hormones are secreted by thyrocytes through fluid phase uptake and lysosomal degradation of the prohormone thyroglobulin (Tg). Hyperthyroidism occurs when there is an overproduction of these thyroid hormones.
The body has a negative-feedback pathway that controls hyperthyroidism (refer back to Figure 1). Immune system signaling is important for hormone synthesis to be controlled. Consider immune system signaling in assisting TSH's up-regulation of hormone synthesis and in turn TSH's down-regulation by thyroid hormones themselves. This cycle is what helps stablize hyperthyroidism from occurring in less susceptible individuals (Lisi et al., 2003).
Graves’ disease can occur in genetically susceptible individuals when an environmental factor, possibly an infectious agent damages the thyroid leading to the exposure of self-antigens to the immune system. Infectious agents have been considered an important environmental inducer of autoimmune diseases. For example, Yersinia Enterocolitica is a common intestinal parasite, which may induce reactivity to TSH receptor. Within the pathogenic bacteria, the TSH receptor could actually appear to be coded for by the bacteria’s plasmids. This concept can be referred as molecular mimicry. A substantial amount of individuals who are affected by Graves’ disease have been infected with this bacteria compared to people who do not have Graves' disease. If this bacterium is exposed to your body as well as your immune system, it can induce TSH receptor antibodies and TSH would appear to bind to a molecule on the bacteria leading to an overproduction of thyroid-stimulating hormones (DeGroot et al., 2005).
Certain cells such as lymphocytes and thyroid can further increase autoimmune responses in Graves' disease. Thyroid cells are able to interact with the immune system causing a response where the symptoms of hyperthyroidism worsen. Unfortunately, thyrocytes that make up the thyroid follicle express and synthesize certain proteins that they normally would not (Weetman et al., 2000). When there is an over-production of thyroid hormones thyroid-reactive T cells are formed and they infiltrate the thyroid gland. In Graves' disease, the majority of the T cells differentiate into Th2 instead of Th1 cells where they can activate B cells which produce TSHR antibodies. This differentiation into Th2 cells allows the stimulation of the thyroid, which causes clinical hyperthyroidism (Davies et al., 2000). In contrast, Hashimoto thyroiditis occurs in the opposite direction as Graves' disease by infilrating T cells differentiating into Th1 cells, which thyroid follicular cells undergo apoptosis leading to clinical hypothyroidism (underactivity/failure of the thyroid) (Weetman et al., 1996).
--------------------------------------------------------------------------------
Current Treatments of Graves' Disease
People who develop this autoimmune disease try to treat it with one of the three different type of treatments such as anti-thyroid drugs, radioactive iodine or surgical thyroid removal. Radioactive idoine is the most common treatment for Graves' disease in North America.
Anti-thyroid drugs can decrease the production of excessive thyroid hormones produced by the thyroid, sustaining thyroid hormone synthesis back to its threshold. Radioactive iodine decreases thyroid hormone levels by damaging thyroid cells, which eventually shrinks the cells leading to their destruction and as well as the functioning of the thyroid gland. Surgical treatment is the removal of the entire thyroid gland, which in many cases people develop an under-active thyroid. If an individual decides to take the surgical route they will have to take thyroid replacement hormones for the duration of their life (National Women's Health Information Center et al., 2006).
The main anti-thyroid drug used in the United States is called methimazole (Wikipedia contributors et al., 2006). “Methimazole…restores euthyroidism by inhibiting thyroperoxidase activity and hormone formation within Tg" (Lisi et al., 2003).
الثلاثاء، 31 مارس 2009
Cardiac catheterization
Radiocontrast in the left ventricle during cardiac catheterization (ventriculogram).
Visualization of the coronary arteries (in this case, the left coronary artery which gives the circumflex branch of left coronary artery).
Cardiac catheterization (heart cath) is the insertion of a catheter into a chamber or vessel of the heart. This is done for both investigational and interventional purposes. Coronary catheterization is a subset of this technique, involving the catheterization of the coronary arteries.
A small puncture is made in a vessel in the groin, the inner bend of the elbow, or neck area (the femoral vessels or the carotid/jugular vessels), then a guidewire is inserted into the incision and threaded through the vessel into the area of the heart that requires treatment, visualized by fluoroscopy or echocardiogram, and a catheter is then threaded over the guidewire. If X-ray fluoroscopy is used, a radiocontrast agent will be administered to the patient during the procedure. When the necessary procedures are complete, the catheter is removed. Firm pressure is applied to the site to prevent bleeding. This may be done by hand or with a mechanical device. Other closure techniques include an internal suture. If the femoral artery was used, the patient will probably be asked to lie flat for several hours to prevent bleeding or the development of a hematoma. Cardiac interventions such as the insertion of a stent prolong both the procedure itself as well as the post-catheterization time spent in allowing the wound to clot.
A cardiac catheterization is a general term for a group of procedures that are performed using this method, such as coronary angiography, as well as left ventrical angiography. Once the catheter is in place, it can be used to perform a number of procedures including angioplasty, angiography, and balloon septostomy.
History
Main article: History of invasive and interventional cardiology
The history of cardiac catheterization dates back to Claude Bernard (1813-1878), who used it on animal models. Clinical application of cardiac catheterization begins with Werner Forssmann in the 1930s, who inserted a catheter into the vein of his own forearm, guided it fluoroscopically into his right atrium, and took an X-ray picture of it. Forssmann won the Nobel Prize in Physiology or Medicine for this achievement. During World War II, André Frédéric Cournand and his colleagues developed techniques for left and right heart catheterization. Indications for investigational use
This technique has several goals:
• confirm the presence of a suspected heart ailment
• quantify the severity of the disease and its effect on the heart
• seek out the cause of a symptom such as shortness of breath or signs of cardiac insufficiency
• make a patient assessment prior to heart surgery
Investigative techniques used with cardiac catheterization
A probe that is opaque to X-rays is inserted into the left or right chambers of the heart for the following reasons:
• to measure intracardiac and intravascular blood pressures
• to take tissue samples for biopsy
• to inject various agents for measuring blood flow in the heart; also to detect and quantify the presence of an intracardiac shunt
• to inject contrast agents in order to study the shape of the heart vessels and chambers and how they change as the heart beats
Radiocontrast in the left ventricle during cardiac catheterization (ventriculogram).
Visualization of the coronary arteries (in this case, the left coronary artery which gives the circumflex branch of left coronary artery).
Cardiac catheterization (heart cath) is the insertion of a catheter into a chamber or vessel of the heart. This is done for both investigational and interventional purposes. Coronary catheterization is a subset of this technique, involving the catheterization of the coronary arteries.
A small puncture is made in a vessel in the groin, the inner bend of the elbow, or neck area (the femoral vessels or the carotid/jugular vessels), then a guidewire is inserted into the incision and threaded through the vessel into the area of the heart that requires treatment, visualized by fluoroscopy or echocardiogram, and a catheter is then threaded over the guidewire. If X-ray fluoroscopy is used, a radiocontrast agent will be administered to the patient during the procedure. When the necessary procedures are complete, the catheter is removed. Firm pressure is applied to the site to prevent bleeding. This may be done by hand or with a mechanical device. Other closure techniques include an internal suture. If the femoral artery was used, the patient will probably be asked to lie flat for several hours to prevent bleeding or the development of a hematoma. Cardiac interventions such as the insertion of a stent prolong both the procedure itself as well as the post-catheterization time spent in allowing the wound to clot.
A cardiac catheterization is a general term for a group of procedures that are performed using this method, such as coronary angiography, as well as left ventrical angiography. Once the catheter is in place, it can be used to perform a number of procedures including angioplasty, angiography, and balloon septostomy.
History
Main article: History of invasive and interventional cardiology
The history of cardiac catheterization dates back to Claude Bernard (1813-1878), who used it on animal models. Clinical application of cardiac catheterization begins with Werner Forssmann in the 1930s, who inserted a catheter into the vein of his own forearm, guided it fluoroscopically into his right atrium, and took an X-ray picture of it. Forssmann won the Nobel Prize in Physiology or Medicine for this achievement. During World War II, André Frédéric Cournand and his colleagues developed techniques for left and right heart catheterization. Indications for investigational use
This technique has several goals:
• confirm the presence of a suspected heart ailment
• quantify the severity of the disease and its effect on the heart
• seek out the cause of a symptom such as shortness of breath or signs of cardiac insufficiency
• make a patient assessment prior to heart surgery
Investigative techniques used with cardiac catheterization
A probe that is opaque to X-rays is inserted into the left or right chambers of the heart for the following reasons:
• to measure intracardiac and intravascular blood pressures
• to take tissue samples for biopsy
• to inject various agents for measuring blood flow in the heart; also to detect and quantify the presence of an intracardiac shunt
• to inject contrast agents in order to study the shape of the heart vessels and chambers and how they change as the heart beats
الاثنين، 9 مارس 2009
Respiratory disease
Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is a group of diseases of the lungs in which the airways become narrowed. This leads to a limitation of the flow of air to and from the lungs causing shortness of breath. In contrast to asthma, the limitation of airflow is poorly reversible and usually gets progressively worse over time.
COPD is caused by noxious particles or gases, most commonly from smoking, which trigger an abnormal inflammatory response in the lung.[1][2] The inflammatory response in the larger airways is known as chronic bronchitis, which is diagnosed clinically when people regularly cough up sputum. In the alveoli, the inflammatory response causes destruction of the tissue of the lung, a process known as emphysema. The natural course of COPD is characterized by occasional sudden worsenings of symptoms called acute exacerbations, most of which are caused by infections or air pollution.
The diagnosis of COPD requires lung function tests. Important management strategies are smoking cessation, vaccinations, rehabilitation, and drug therapy (often using inhalers). Some patients go on to requiring long-term oxygen therapy or lung transplantation.[1]
Worldwide, COPD ranked sixth as the cause of death in 1990. It is projected to be the third leading cause of death worldwide by 2020 due to an increase in smoking rates and demographic changes in many countries.[1] COPD is the 4th leading cause of death in the U.S., and the economic burden of COPD in the U.S. in 2007 was $42.6 billion in health care costs and lost productivity.[3][4]
COPD is also known as chronic obstructive lung disease (COLD), chronic obstructive airway disease (COAD), chronic airflow limitation (CAL) and chronic obstructive respiratory disease.
[edit] Signs and symptoms
One of the most common symptoms of COPD is shortness of breath (dyspnea). People with COPD commonly describe this as: “My breathing requires effort”, “I feel out of breath”, or “I can not get enough air in”.[5] People with COPD typically first notice dyspnea during vigorous exercise when the demands on the lungs are greatest. Over the years, dyspnea tends to get gradually worse so that it can occur during milder, everyday activities such as housework. In the advanced stages of COPD, dyspnea can become so bad that it occurs during rest and is constantly present. Other symptoms of COPD are a persistent cough, sputum or mucus production, wheezing, chest tightness, and tiredness.[6][7] People with advanced (very severe) COPD sometimes develop respiratory failure. When this happens, cyanosis, a bluish discoloration of the lips caused by a lack of oxygen in the blood, can occur. An excess of carbon dioxide in the blood can cause headaches, drowsiness or twitching (asterixis). A complication of advanced COPD is cor pulmonale, a strain on the heart due to the extra work required by the heart to pump blood through the affected lungs.[8] Symptoms of cor pulmonale are peripheral edema, seen as swelling of the ankles, and dyspnea.
There are a few signs of COPD that a healthcare worker may detect although they can be seen in other diseases. Some people have COPD and have none of these signs. Common signs are:
• tachypnea, a rapid breathing rate
• wheezing sounds or crackles in the lungs heard through a stethoscope
• breathing out taking a longer time than breathing in
• enlargement of the chest, particularly the front-to-back distance (hyperinflation)
• active use of muscles in the neck to help with breathing
• breathing through pursed lips
• increased anteroposterior to lateral ratio of the chest (i.e. barrel chest)
Etiology
Smoking
The primary risk factor for COPD is chronic tobacco smoking. In the United States, 80 to 90% of cases of COPD are due to smoking.[9] Exposure to cigarette smoke is measured in pack-years, the average number of packages of cigarettes smoked daily multiplied by the number of years of smoking. Not all smokers will develop COPD, but continuous smokers have at least a 25% risk after 25 years.[10] The likelihood of developing COPD increases with increasing age as the cumulative smoke exposure increases. Inhaling the smoke from other peoples' cigarettes (passive smoking) can lead to impaired lung growth and could be a cause of COPD.
Occupational exposures
Intense and prolonged exposure to workplace dusts found in coal mining, gold mining, and the cotton textile industry and chemicals such as cadmium, isocyanates, and fumes from welding have been implicated in the development of airflow obstruction, even in nonsmokers.[11] Workers who smoke and are exposed to these particles and gases are even more likely to develop COPD. Intense silica dust exposure causes silicosis, a restrictive lung disease distinct from COPD; however, less intense silica dust exposures have been linked to a COPD-like condition.[12] The effect of occupational pollutants on the lungs appears to be substantially less important than the effect of cigarette smoking.[13]
Air pollution
Studies in many countries have found that people who live in large cities have a higher rate of COPD compared to people who live in rural areas[14]. Urban air pollution may be a contributing factor for COPD as it is thought to slow the normal growth of the lungs although the long-term research needed to confirm the link has not been done. In many developing countries indoor air pollution from cooking fire smoke (often using biomass fuels such as wood and animal dung) is a common cause of COPD, especially in women[15].
Genetics
Only about half of all long-term smokers will ever develop COPD. Some factor in addition to heavy smoke exposure is required for a person to develop COPD. This factor is probably a genetic susceptibility. COPD is more common among relatives of COPD patients who smoke than unrelated smokers.[16] The genetic differences that make some peoples' lungs susceptible to the effects of tobacco smoke are mostly unknown. Alpha 1-antitrypsin deficiency is a genetic condition that is responsible for about 2% of cases of COPD. In this condition, the body does not make enough of a protein, alpha 1-antitrypsin. Alpha 1-antitrypsin protects the lungs from damage caused by protease enzymes, such as trypsin, that can be released as a result of an inflammatory response to tobacco smoke.[17]
Other risk factors
A tendency to sudden airway constriction in response to inhaled irritants, bronchial hyperresponsiveness, is a characteristic of asthma. Many people with COPD also have this tendency. In COPD, the presence of bronchial hyperresponsiveness predicts a worse course of the disease.[13] It is not known if bronchial hyperresponsiveness is a cause or a consequence of COPD. Other risk factors such as repeated lung infection and possibly a diet high in cured meats may be related to the development of COPD.
COPD as an autoimmune disease
Main article: Autoimmunity
There is mounting evidence that there may be an autoimmune component to COPD. Many individuals with COPD who have stopped smoking have active inflammation in the lungs. The disease may continue to get worse for many years after stopping smoking due to this ongoing inflammation. This sustained inflammation is thought to be mediated by autoantibodies and autoreactive T cells.[18][19][20]
Disease process
Enlarged view of lung tissue showing the difference between healthy lung and COPD
It is not fully understood how tobacco smoke and other inhaled particles damage the lungs to cause COPD. The most important processes causing lung damage are:
• Oxidative stress produced by the high concentrations of free radicals in tobacco smoke.
• Cytokine release due to inflammation as the body responds to irritant particles such as tobacco smoke in the airway.
Pathology
• Tobacco smoke and free radicals impair the activity of antiprotease enzymes such as alpha 1-antitrypsin, allowing protease enzymes to damage the lung.
Chronic bronchitis
Main article: chronic bronchitis
Lung damage and inflammation in the large airways results in chronic bronchitis. Chronic bronchitis is defined in clinical terms as a cough with sputum production on most days for 3 months of a year, for 2 consecutive years[21]. In the airways of the lung, the hallmark of chronic bronchitris is an increased number (hyperplasia) and increased size (hypertrophy) of the goblet cells and mucous glands of the airway. As a result, there is more mucus than usual in the airways, contributing to narrowing of the airways and causing a cough with sputum. Microscopically there is infiltration of the airway walls with inflammatory cells. Inflammation is followed by scarring and remodeling that thickens the walls and also results in narrowing of the airways. As chronic bronchitis progresses, there is squamous metaplasia (an abnormal change in the tissue lining the inside of the airway) and fibrosis (further thickening and scarring of the airway wall). The consequence of these changes is a limitation of airflow[22].
Patients with advanced COPD that have primarily chronic bronchitis rather than emphysema were commonly referred to as "blue bloaters" because of the bluish color of the skin and lips (cyanosis) seen in them.[23]
Emphysema
Gross pathology of a lung showing centrilobular-type emphysema characteristic of smoking. This close-up of the fixed, cut lung surface shows multiple cavities lined by heavy black carbon deposits.
Main article: emphysema
Lung damage and inflammation of the air sacs (alveoli) results in emphysema. Emphysema is defined as enlargement of the air spaces distal to the terminal bronchioles, with destruction of their walls.[21] The destruction of air space walls reduces the surface area available for the exchange of oxygen and carbon dioxide during breathing. It also reduces the elasticity of the lung itself, which results in a loss of support for the airways that are embedded in the lung. These airways are more likely to collapse causing further limitation to airflow. The effort made by patients suffering from emphysema during exhalation, causes a pink color in their faces, hence the term commonly used to refer to them, "pink puffers".
Pathophysiology
Narrowing of the airways reduces the rate at which air can flow to and from the air sacs (alveoli) and limits the effectiveness of the lungs. In COPD, the greatest reduction in air flow occurs when breathing out (during expiration) because the pressure in the chest tends to compress rather than expand the airways. In theory, air flow could be increased by breathing more forcefully, increasing the pressure in the chest during expiration. In COPD, there is often a limit to how much this can actually increase air flow, a situation known as expiratory flow limitation[24].
If the rate of airflow is too low, a person with COPD may not be able to completely finish breathing out (expiration) before he or she needs to take another breath. This is particularly common during exercise when breathing has to be faster. A little of the air of the previous breath remains within the lungs when the next breath is started. When this happens, there is an increase in the volume of air in the lungs, a process called dynamic hyperinflation.[24]
Dynamic hyperinflation is closely linked to shortness of breath (dyspnea) in COPD[25]. It is less comfortable to breathe with hyperinflation because it takes more effort to move the lungs and chest wall when they are already stretched by hyperinflation.
Another factor contributing to shortness of breath in COPD is the loss of the surface area available for the exchange of oxygen and carbon dioxide with emphysema. This reduces the rate of transfer of these gasses between the body and the atmosphere and can lead to low oxygen and high carbon dioxide levels in the body. A person with emphysema may have to breathe faster or more deeply to compensate, which can be difficult to do if there is also flow limitation or hyperinflation.
Some people with advanced COPD do manage to breathe fast to compensate, but usually have dyspnea as a result. Others, who may be less short of breath, tolerate low oxygen and high carbon dioxide levels in their bodies but this can eventually lead to headaches, drowsiness and heart failure.
Advanced COPD can lead to complications beyond the lungs such as weight loss (cachexia), pulmonary hypertension and heart failure (cor pulmonale). Osteoporosis, heart disease, muscle wasting and depression are all more common in people with COPD[1].
Acute exacerbations of COPD
An acute exacerbation of COPD is a sudden worsening of COPD symptoms (shortness of breath, quantity and color of phlegm) that typically lasts for several days. It may be triggered by an infection with bacteria or viruses or by environmental pollutants. Typically, infections cause 75% or more of the exacerbations; bacteria can roughly be found in 25% of cases, viruses in another 25%, and both viruses and bacteria in another 25%. Pulmonary Embolism can also cause exacerbations of COPD. Airway inflammation is increased during the exacerbation resulting in increased hyperinflation, reduced expiratory air flow and worsening of gas transfer.[1]
Diagnosis
The diagnosis of COPD should be considered in anyone who has dyspnea, chronic cough or sputum production, and/or a history of exposure to risk factors for the disease such as regular tobacco smoking[1][26]. No single symptom or sign can adequately confirm or exclude the diagnosis of COPD[27] although COPD is uncommon under the age of 40 years.
Spirometry
The diagnosis of COPD is confirmed by spirometry[1], a test that measures breathing. Spirometry measures the forced expiratory volume in one second (FEV1) which is the greatest volume of air that can be breathed out in the first second of a large breath. Spirometry also measures the forced vital capacity (FVC) which is the greatest volume of air that can be breathed out in a whole large breath. Normally at least 70% of the FVC comes out in the first second (i.e. the FEV1/FVC ratio is >70%). In COPD, this ratio is less than normal, (i.e. FEV1/FVC ratio is <70%) even after a bronchodilator medication has been given.
Spirometry can help to determine the severity of COPD.[1] The FEV1 (measured post-bronchodilator) is expressed as a percent of a predicted "normal" value based on a person's age, gender, height and weight:
Severity of COPD FEV1 % predicted
Mild ≥80
Moderate 50-79
Severe 30-49
Very severe <30 or Chronic respiratory failure symptoms
The severity of COPD also depends on the severity of dyspnea and exercise limitation. These and other factors can be combined with spirometry results to obtain a COPD severity score that takes multiple dimensions of the disease into account[28].
Other tests
An x-ray of the chest may show an over-expanded lung (hyperinflation) and can be useful to help exclude other lung diseases. Complete pulmonary function tests with measurements of lung volumes and gas transfer may also show hyperinflation and can discriminate between COPD with emphysema and COPD without emphysema. A high-resolution computed tomography scan of the chest may show the distribution of emphysema throughout the lungs and can also be useful to exclude other lung diseases.
A blood sample taken from an artery can be tested for blood gas levels which may show low oxygen levels (hypoxemia) and/or high carbon dioxide levels (respiratory acidosis). A blood sample taken from a vein may show a high blood count (reactive polythycaemia), a reaction to long-term hypoxemia.
Management
There is currently no cure for COPD; however, COPD is both a preventable and treatable disease Clinical practice guidelines for the management of COPD are available from the Global Initiative for Chronic Obstructive Lung Disease (GOLD),[29] a collaboration that includes the World Health Organization and the U.S. National Heart, Lung, and Blood Institute. The major current directions of COPD management are to assess and monitor the disease, reduce the risk factors, manage stable COPD, prevent and treat acute exacerbations and manage comorbidity.[1]
Risk factor reduction
Smoking cessation
Main article: Smoking cessation
Smoking cessation is one of the most important factors in slowing down the progression of COPD. Once COPD has been diagnosed, stopping smoking slows down the rate of progression of the disease. Even at a late stage of the disease it can significantly reduce the rate of deterioration in lung function and delay the onset of disability and death.[22] It is the only standard intervention that can improve the rate of progression of COPD.
Smoking cessation starts with an individual decision to stop smoking that leads to an attempt at quitting. Often several attempts are required before long-term smoking cessation is achieved[30]. Some smokers can achieve long-term smoking cessation through "willpower" alone. However smoking is highly addictive and many smokers need further support to quit. The chance of successfully stopping smoking can be greatly improved through social support, engagement in a smoking cessation programme and the use of drugs such as nicotine replacement therapy, bupropion and varenicline.[30]
The policies of governments, public health agencies and anti-smoking organizations can reduce smoking rates by encouraging smoking cessation and discouraging people from starting smoking.[30] These policies are important strategies in the prevention of COPD.
Occupational health
Measures can be taken to reduce the likelihood that workers in at-risk industries such as coal mining will develop COPD. Some examples of these measures are: education of workers and management about the risks, promoting smoking cessation, surveillance of workers for early signs of COPD, the use of personal dust monitors, the use of respirators and dust control[31]. Dust control can be achieved by improving ventilation, using water sprays and by using mining techniques that minimize dust generation. If a worker develops COPD, further lung damage can be reduced by avoiding ongoing dust exposure, for example by changing the work role.
Air pollution
Air quality can be improved by pollution reduction efforts which should lead to health gains for people with COPD. A person who has COPD may experience fewer symptoms if they stay indoors on days when air quality is poor.[1]
Management of stable COPD
Bronchodilators
Bronchodilators are medicines that relax smooth muscle around the airways, increasing the calibre of the airways and improving air flow. They can reduce the symptoms of shortness of breath, wheeze and exercise limitation, resulting in an improved quality of life for people with COPD. [32] They do not slow down the rate of progression of the underlying disease.[1] Bronchodilators are usually administered with an inhaler or via a nebulizer.
There are two major types of bronchodilator, β2 agonists and anticholinergics. Anticholinergics appear to be superior to β2 agonists in COPD. Anticholinergeics reduce repiratory deaths while β2 agonists have no effect on respiratory deaths.[33] Each type may be either long-acting (with an effect lasting 12 hours or more) or short-acting (with a rapid onset of effect that does not last as long).
β2 agonists
β2 agonists stimulate β2 receptors on airway smooth muscles, causing them to relax. There are several β2 agonists available. Salbutamol or albuterol (common brand name: Ventolin) and terbutaline are widely used short acting β2 agonists and provide rapid relief of COPD symptoms. Long acting β2 agonists (LABAs) such as salmeterol and formoterol are used as maintenance therapy and lead to improved airflow, exercise capacity, quality of life and possibly a longer life.[34]
Anticholinergics
Anticholinergic drugs cause airway smooth muscles to relax by blocking stimulation from cholinergic nerves. Ipratropium is the most widely prescribed short acting anticholinergic drug. Like short-acting β2 agonists, short-acting anticholinergics provide rapid relief of COPD symptoms and a combination of the two is commonly used for a greater bronchodilator effect. Tiotropium is the most commonly prescribed long-acting anticholinergic drug in COPD. It is has more specificity for M3 muscarinic receptors so may have less side-effects than other anticholinergic drugs. Regular use is associated with improvements in airflow, exercise capacity, quality of life and possibly a longer life. [35] [36]
Corticosteroids
Corticosteroids act to reduce the inflammation in the airways, in theory reducing lung damage and airway narrowing caused by inflammation.[37] Unlike bronchodilators, they do not act directly on the airway smooth muscle and do not provide immediate relief of symptoms. Some of the more common corticosteroids in use are prednisone, fluticasone, budesonide, mometasone, and beclomethasone. Corticosteroids are used in tablet or inhaled form to treat and prevent acute exacerbations of COPD. Inhaled corticosteroids have not been shown to be of benefit for people with mild COPD however they are beneficial for those with either moderate of severe COPD.[38] Most people with COPD who use inhaled corticosteroids also use a long-acting bronchodilator so inhaled corticosteroids are often combined with a LABA in the same inhaler.[citation needed]
Other medication
Theophylline is a bronchodilator and phosphodiesterase inhibitor that in high doses can reduce symptoms for some people who have COPD. More often, side effects such as nausea and stimulation of the heart limit its use.[1] In lower doses, it may slightly reduce the number of COPD exacerbations.[39] The investigative phosphodiesterase-4 antagonists, roflumilast and cilomilast have completed Phase-2 clinical trials. Tumor necrosis factor antagonists such as infliximab suppress the immune system and reduce inflammation. Infliximab has been trialled in COPD but there was no evidence of benefit with the possibility of harm.[40]
Supplemental oxygen
Oxygen can be delivered in different forms: in large containers, in smaller containers with liquid oxygen, or with the use of a oxygen concentrator (shown here) which derives oxygen from room air. The latter two options improve mobility of people requiring long-term oxygen therapy.
Supplemental oxygen can be given to people with COPD who have low oxygen levels in the body. Oxygen is provided from an oxygen cylinder or an oxygen concentrator and delivered to a person through tubing via a nasal cannula or oxygen mask. Supplemental oxygen does not greatly improve shortness of breath but can allow people with COPD and low oxygen levels to do more exercise and household activity. Long-term oxygen therapy for at least 16 hours a day can improve the quality of life and survival for people with COPD and arterial hypoxemia or with complications of hypoxemia such as pulmonary hypertension, cor pulmonale, or secondary erythrocytosis.[41] High concentrations of supplemental oxygen can lead to the accumulation of carbon dioxide and respiratory acidosis for some people with severe COPD; lower oxygen flow rates are generally safer for these individuals.
Pulmonary rehabilitation
Pulmonary rehabilitation is a program of exercise, disease management and counselling coordinated to benefit the individual.[42] Pulmonary rehabilitation has been shown to improve shortness of breath and exercise capacity. It has also been shown to improve the sense of control a patient has over their disease as well as their emotions.[43]
Nutrition
Being either underweight or overweight can affect the symptoms, degree of disability and prognosis of COPD. People with COPD who are underweight can improve their breathing muscle strength by increasing their calorie intake.[1] When combined with regular exercise or a pulmonary rehabilitation programme, this can lead to improvements in COPD symptoms.
Surgery
Surgery is sometimes helpful for COPD in selected cases. A bullectomy is the surgical removal of a bulla, a large air-filled space that can squash the surrounding, more normal lung. Lung volume reduction surgery is similar; parts of the lung that are particularly damaged by emphysema are removed allowing the remaining, relatively good lung to expand and work better. Lung transplantation is sometimes performed for severe COPD, particularly in younger individuals.
Management of acute exacerbations
Acute exacerbations can be partially prevented. Infections are responsible for approximately half of COPD exacerbations, some of which can be prevented by vaccination against pathogens such as influenza and Streptococcus pneumoniae. Regular medication use can prevent some COPD exacerbations; LABAs, long-acting anticholinergics, inhaled corticosteroids and low-dose theophylline have all been shown to reduce the frequency of COPD exacerbations.[34] [35][44][39] The symptoms of acute exacerbations are treated using short-acting bronchodilators. A course of corticosteroids, usually in tablet or intravenous rather than inhaled form, can speed up recovery. [1] Antibiotics are often used but will only help if the exacerbation is due to an infection.[45] Antibiotics are indicated when a patient notes sputum changes, has an elevated white count, or is febrile. Severe exacerbations can require hospital care where treatments such as oxygen and mechanical ventilation may be required.[46]
Prognosis
COPD usually gradually gets worse over time and can lead to death. The rate at which it gets worse varies between individuals. The factors that predict a poorer prognosis are: [1]
• Severe airflow obstruction (low FEV1)
• Poor exercise capacity
• Shortness of breath
• Significantly underweight or overweight
• Complications like respiratory failure or cor pulmonale
• Continued smoking
• Frequent acute exacerbations
History
COPD has probably always existed but has been called by different names in the past. Bonet described a condition of “voluminous lungs” in 1679. In 1769,Giovanni Morgagni described 19 cases where the lungs were “turgid” particularly from air.[47] The first description and illustration of the enlarged airspaces in emphysema was provided by Ruysh in 1721."History of pathologic descriptions of COPD" (PDF). http://www.mhprofessional.com/downloads/products/0071457399/0071457399_chap40.pdf. Matthew Baillie illustrated an emphysematous lung in 1789 and described the destructive character of the condition.[47] Badham used the word "catarrh" to describe the cough and mucus hypersecretion of chronic bronchitis in 1814. He recognised that chronic bronchitis was a disabling disorder.
René Laennec, the physician who invented the stethoscope, used the term emphysema in his 1837 book A treatise on the diseases of the chest and of mediate auscultation to describe lungs that did not collapse when he opened the chest during an autopsy. He noted that they did not collapse as usual because they were full of air and the airways were filled with mucus.[47]
John Hutchinson invented the spirometer in 1842 that allowed the measurement of vital airflow.[48] Tiffeneau in 1947 and Gaensler in 1950 and 1951 described the principles of measuring airflow.capacity of the lungs however his spirometer could only measure volume, not
The terms chronic bronchitis and emphysema were formally defined at the CIBA guest symposium of physicians in 1959. The term COPD was first used by William Briscoe in 1965 and has gradually overtaken other terms to become established today as the preferred name of this disease
Chronic obstructive pulmonary disease (COPD) is a group of diseases of the lungs in which the airways become narrowed. This leads to a limitation of the flow of air to and from the lungs causing shortness of breath. In contrast to asthma, the limitation of airflow is poorly reversible and usually gets progressively worse over time.
COPD is caused by noxious particles or gases, most commonly from smoking, which trigger an abnormal inflammatory response in the lung.[1][2] The inflammatory response in the larger airways is known as chronic bronchitis, which is diagnosed clinically when people regularly cough up sputum. In the alveoli, the inflammatory response causes destruction of the tissue of the lung, a process known as emphysema. The natural course of COPD is characterized by occasional sudden worsenings of symptoms called acute exacerbations, most of which are caused by infections or air pollution.
The diagnosis of COPD requires lung function tests. Important management strategies are smoking cessation, vaccinations, rehabilitation, and drug therapy (often using inhalers). Some patients go on to requiring long-term oxygen therapy or lung transplantation.[1]
Worldwide, COPD ranked sixth as the cause of death in 1990. It is projected to be the third leading cause of death worldwide by 2020 due to an increase in smoking rates and demographic changes in many countries.[1] COPD is the 4th leading cause of death in the U.S., and the economic burden of COPD in the U.S. in 2007 was $42.6 billion in health care costs and lost productivity.[3][4]
COPD is also known as chronic obstructive lung disease (COLD), chronic obstructive airway disease (COAD), chronic airflow limitation (CAL) and chronic obstructive respiratory disease.
[edit] Signs and symptoms
One of the most common symptoms of COPD is shortness of breath (dyspnea). People with COPD commonly describe this as: “My breathing requires effort”, “I feel out of breath”, or “I can not get enough air in”.[5] People with COPD typically first notice dyspnea during vigorous exercise when the demands on the lungs are greatest. Over the years, dyspnea tends to get gradually worse so that it can occur during milder, everyday activities such as housework. In the advanced stages of COPD, dyspnea can become so bad that it occurs during rest and is constantly present. Other symptoms of COPD are a persistent cough, sputum or mucus production, wheezing, chest tightness, and tiredness.[6][7] People with advanced (very severe) COPD sometimes develop respiratory failure. When this happens, cyanosis, a bluish discoloration of the lips caused by a lack of oxygen in the blood, can occur. An excess of carbon dioxide in the blood can cause headaches, drowsiness or twitching (asterixis). A complication of advanced COPD is cor pulmonale, a strain on the heart due to the extra work required by the heart to pump blood through the affected lungs.[8] Symptoms of cor pulmonale are peripheral edema, seen as swelling of the ankles, and dyspnea.
There are a few signs of COPD that a healthcare worker may detect although they can be seen in other diseases. Some people have COPD and have none of these signs. Common signs are:
• tachypnea, a rapid breathing rate
• wheezing sounds or crackles in the lungs heard through a stethoscope
• breathing out taking a longer time than breathing in
• enlargement of the chest, particularly the front-to-back distance (hyperinflation)
• active use of muscles in the neck to help with breathing
• breathing through pursed lips
• increased anteroposterior to lateral ratio of the chest (i.e. barrel chest)
Etiology
Smoking
The primary risk factor for COPD is chronic tobacco smoking. In the United States, 80 to 90% of cases of COPD are due to smoking.[9] Exposure to cigarette smoke is measured in pack-years, the average number of packages of cigarettes smoked daily multiplied by the number of years of smoking. Not all smokers will develop COPD, but continuous smokers have at least a 25% risk after 25 years.[10] The likelihood of developing COPD increases with increasing age as the cumulative smoke exposure increases. Inhaling the smoke from other peoples' cigarettes (passive smoking) can lead to impaired lung growth and could be a cause of COPD.
Occupational exposures
Intense and prolonged exposure to workplace dusts found in coal mining, gold mining, and the cotton textile industry and chemicals such as cadmium, isocyanates, and fumes from welding have been implicated in the development of airflow obstruction, even in nonsmokers.[11] Workers who smoke and are exposed to these particles and gases are even more likely to develop COPD. Intense silica dust exposure causes silicosis, a restrictive lung disease distinct from COPD; however, less intense silica dust exposures have been linked to a COPD-like condition.[12] The effect of occupational pollutants on the lungs appears to be substantially less important than the effect of cigarette smoking.[13]
Air pollution
Studies in many countries have found that people who live in large cities have a higher rate of COPD compared to people who live in rural areas[14]. Urban air pollution may be a contributing factor for COPD as it is thought to slow the normal growth of the lungs although the long-term research needed to confirm the link has not been done. In many developing countries indoor air pollution from cooking fire smoke (often using biomass fuels such as wood and animal dung) is a common cause of COPD, especially in women[15].
Genetics
Only about half of all long-term smokers will ever develop COPD. Some factor in addition to heavy smoke exposure is required for a person to develop COPD. This factor is probably a genetic susceptibility. COPD is more common among relatives of COPD patients who smoke than unrelated smokers.[16] The genetic differences that make some peoples' lungs susceptible to the effects of tobacco smoke are mostly unknown. Alpha 1-antitrypsin deficiency is a genetic condition that is responsible for about 2% of cases of COPD. In this condition, the body does not make enough of a protein, alpha 1-antitrypsin. Alpha 1-antitrypsin protects the lungs from damage caused by protease enzymes, such as trypsin, that can be released as a result of an inflammatory response to tobacco smoke.[17]
Other risk factors
A tendency to sudden airway constriction in response to inhaled irritants, bronchial hyperresponsiveness, is a characteristic of asthma. Many people with COPD also have this tendency. In COPD, the presence of bronchial hyperresponsiveness predicts a worse course of the disease.[13] It is not known if bronchial hyperresponsiveness is a cause or a consequence of COPD. Other risk factors such as repeated lung infection and possibly a diet high in cured meats may be related to the development of COPD.
COPD as an autoimmune disease
Main article: Autoimmunity
There is mounting evidence that there may be an autoimmune component to COPD. Many individuals with COPD who have stopped smoking have active inflammation in the lungs. The disease may continue to get worse for many years after stopping smoking due to this ongoing inflammation. This sustained inflammation is thought to be mediated by autoantibodies and autoreactive T cells.[18][19][20]
Disease process
Enlarged view of lung tissue showing the difference between healthy lung and COPD
It is not fully understood how tobacco smoke and other inhaled particles damage the lungs to cause COPD. The most important processes causing lung damage are:
• Oxidative stress produced by the high concentrations of free radicals in tobacco smoke.
• Cytokine release due to inflammation as the body responds to irritant particles such as tobacco smoke in the airway.
Pathology
• Tobacco smoke and free radicals impair the activity of antiprotease enzymes such as alpha 1-antitrypsin, allowing protease enzymes to damage the lung.
Chronic bronchitis
Main article: chronic bronchitis
Lung damage and inflammation in the large airways results in chronic bronchitis. Chronic bronchitis is defined in clinical terms as a cough with sputum production on most days for 3 months of a year, for 2 consecutive years[21]. In the airways of the lung, the hallmark of chronic bronchitris is an increased number (hyperplasia) and increased size (hypertrophy) of the goblet cells and mucous glands of the airway. As a result, there is more mucus than usual in the airways, contributing to narrowing of the airways and causing a cough with sputum. Microscopically there is infiltration of the airway walls with inflammatory cells. Inflammation is followed by scarring and remodeling that thickens the walls and also results in narrowing of the airways. As chronic bronchitis progresses, there is squamous metaplasia (an abnormal change in the tissue lining the inside of the airway) and fibrosis (further thickening and scarring of the airway wall). The consequence of these changes is a limitation of airflow[22].
Patients with advanced COPD that have primarily chronic bronchitis rather than emphysema were commonly referred to as "blue bloaters" because of the bluish color of the skin and lips (cyanosis) seen in them.[23]
Emphysema
Gross pathology of a lung showing centrilobular-type emphysema characteristic of smoking. This close-up of the fixed, cut lung surface shows multiple cavities lined by heavy black carbon deposits.
Main article: emphysema
Lung damage and inflammation of the air sacs (alveoli) results in emphysema. Emphysema is defined as enlargement of the air spaces distal to the terminal bronchioles, with destruction of their walls.[21] The destruction of air space walls reduces the surface area available for the exchange of oxygen and carbon dioxide during breathing. It also reduces the elasticity of the lung itself, which results in a loss of support for the airways that are embedded in the lung. These airways are more likely to collapse causing further limitation to airflow. The effort made by patients suffering from emphysema during exhalation, causes a pink color in their faces, hence the term commonly used to refer to them, "pink puffers".
Pathophysiology
Narrowing of the airways reduces the rate at which air can flow to and from the air sacs (alveoli) and limits the effectiveness of the lungs. In COPD, the greatest reduction in air flow occurs when breathing out (during expiration) because the pressure in the chest tends to compress rather than expand the airways. In theory, air flow could be increased by breathing more forcefully, increasing the pressure in the chest during expiration. In COPD, there is often a limit to how much this can actually increase air flow, a situation known as expiratory flow limitation[24].
If the rate of airflow is too low, a person with COPD may not be able to completely finish breathing out (expiration) before he or she needs to take another breath. This is particularly common during exercise when breathing has to be faster. A little of the air of the previous breath remains within the lungs when the next breath is started. When this happens, there is an increase in the volume of air in the lungs, a process called dynamic hyperinflation.[24]
Dynamic hyperinflation is closely linked to shortness of breath (dyspnea) in COPD[25]. It is less comfortable to breathe with hyperinflation because it takes more effort to move the lungs and chest wall when they are already stretched by hyperinflation.
Another factor contributing to shortness of breath in COPD is the loss of the surface area available for the exchange of oxygen and carbon dioxide with emphysema. This reduces the rate of transfer of these gasses between the body and the atmosphere and can lead to low oxygen and high carbon dioxide levels in the body. A person with emphysema may have to breathe faster or more deeply to compensate, which can be difficult to do if there is also flow limitation or hyperinflation.
Some people with advanced COPD do manage to breathe fast to compensate, but usually have dyspnea as a result. Others, who may be less short of breath, tolerate low oxygen and high carbon dioxide levels in their bodies but this can eventually lead to headaches, drowsiness and heart failure.
Advanced COPD can lead to complications beyond the lungs such as weight loss (cachexia), pulmonary hypertension and heart failure (cor pulmonale). Osteoporosis, heart disease, muscle wasting and depression are all more common in people with COPD[1].
Acute exacerbations of COPD
An acute exacerbation of COPD is a sudden worsening of COPD symptoms (shortness of breath, quantity and color of phlegm) that typically lasts for several days. It may be triggered by an infection with bacteria or viruses or by environmental pollutants. Typically, infections cause 75% or more of the exacerbations; bacteria can roughly be found in 25% of cases, viruses in another 25%, and both viruses and bacteria in another 25%. Pulmonary Embolism can also cause exacerbations of COPD. Airway inflammation is increased during the exacerbation resulting in increased hyperinflation, reduced expiratory air flow and worsening of gas transfer.[1]
Diagnosis
The diagnosis of COPD should be considered in anyone who has dyspnea, chronic cough or sputum production, and/or a history of exposure to risk factors for the disease such as regular tobacco smoking[1][26]. No single symptom or sign can adequately confirm or exclude the diagnosis of COPD[27] although COPD is uncommon under the age of 40 years.
Spirometry
The diagnosis of COPD is confirmed by spirometry[1], a test that measures breathing. Spirometry measures the forced expiratory volume in one second (FEV1) which is the greatest volume of air that can be breathed out in the first second of a large breath. Spirometry also measures the forced vital capacity (FVC) which is the greatest volume of air that can be breathed out in a whole large breath. Normally at least 70% of the FVC comes out in the first second (i.e. the FEV1/FVC ratio is >70%). In COPD, this ratio is less than normal, (i.e. FEV1/FVC ratio is <70%) even after a bronchodilator medication has been given.
Spirometry can help to determine the severity of COPD.[1] The FEV1 (measured post-bronchodilator) is expressed as a percent of a predicted "normal" value based on a person's age, gender, height and weight:
Severity of COPD FEV1 % predicted
Mild ≥80
Moderate 50-79
Severe 30-49
Very severe <30 or Chronic respiratory failure symptoms
The severity of COPD also depends on the severity of dyspnea and exercise limitation. These and other factors can be combined with spirometry results to obtain a COPD severity score that takes multiple dimensions of the disease into account[28].
Other tests
An x-ray of the chest may show an over-expanded lung (hyperinflation) and can be useful to help exclude other lung diseases. Complete pulmonary function tests with measurements of lung volumes and gas transfer may also show hyperinflation and can discriminate between COPD with emphysema and COPD without emphysema. A high-resolution computed tomography scan of the chest may show the distribution of emphysema throughout the lungs and can also be useful to exclude other lung diseases.
A blood sample taken from an artery can be tested for blood gas levels which may show low oxygen levels (hypoxemia) and/or high carbon dioxide levels (respiratory acidosis). A blood sample taken from a vein may show a high blood count (reactive polythycaemia), a reaction to long-term hypoxemia.
Management
There is currently no cure for COPD; however, COPD is both a preventable and treatable disease Clinical practice guidelines for the management of COPD are available from the Global Initiative for Chronic Obstructive Lung Disease (GOLD),[29] a collaboration that includes the World Health Organization and the U.S. National Heart, Lung, and Blood Institute. The major current directions of COPD management are to assess and monitor the disease, reduce the risk factors, manage stable COPD, prevent and treat acute exacerbations and manage comorbidity.[1]
Risk factor reduction
Smoking cessation
Main article: Smoking cessation
Smoking cessation is one of the most important factors in slowing down the progression of COPD. Once COPD has been diagnosed, stopping smoking slows down the rate of progression of the disease. Even at a late stage of the disease it can significantly reduce the rate of deterioration in lung function and delay the onset of disability and death.[22] It is the only standard intervention that can improve the rate of progression of COPD.
Smoking cessation starts with an individual decision to stop smoking that leads to an attempt at quitting. Often several attempts are required before long-term smoking cessation is achieved[30]. Some smokers can achieve long-term smoking cessation through "willpower" alone. However smoking is highly addictive and many smokers need further support to quit. The chance of successfully stopping smoking can be greatly improved through social support, engagement in a smoking cessation programme and the use of drugs such as nicotine replacement therapy, bupropion and varenicline.[30]
The policies of governments, public health agencies and anti-smoking organizations can reduce smoking rates by encouraging smoking cessation and discouraging people from starting smoking.[30] These policies are important strategies in the prevention of COPD.
Occupational health
Measures can be taken to reduce the likelihood that workers in at-risk industries such as coal mining will develop COPD. Some examples of these measures are: education of workers and management about the risks, promoting smoking cessation, surveillance of workers for early signs of COPD, the use of personal dust monitors, the use of respirators and dust control[31]. Dust control can be achieved by improving ventilation, using water sprays and by using mining techniques that minimize dust generation. If a worker develops COPD, further lung damage can be reduced by avoiding ongoing dust exposure, for example by changing the work role.
Air pollution
Air quality can be improved by pollution reduction efforts which should lead to health gains for people with COPD. A person who has COPD may experience fewer symptoms if they stay indoors on days when air quality is poor.[1]
Management of stable COPD
Bronchodilators
Bronchodilators are medicines that relax smooth muscle around the airways, increasing the calibre of the airways and improving air flow. They can reduce the symptoms of shortness of breath, wheeze and exercise limitation, resulting in an improved quality of life for people with COPD. [32] They do not slow down the rate of progression of the underlying disease.[1] Bronchodilators are usually administered with an inhaler or via a nebulizer.
There are two major types of bronchodilator, β2 agonists and anticholinergics. Anticholinergics appear to be superior to β2 agonists in COPD. Anticholinergeics reduce repiratory deaths while β2 agonists have no effect on respiratory deaths.[33] Each type may be either long-acting (with an effect lasting 12 hours or more) or short-acting (with a rapid onset of effect that does not last as long).
β2 agonists
β2 agonists stimulate β2 receptors on airway smooth muscles, causing them to relax. There are several β2 agonists available. Salbutamol or albuterol (common brand name: Ventolin) and terbutaline are widely used short acting β2 agonists and provide rapid relief of COPD symptoms. Long acting β2 agonists (LABAs) such as salmeterol and formoterol are used as maintenance therapy and lead to improved airflow, exercise capacity, quality of life and possibly a longer life.[34]
Anticholinergics
Anticholinergic drugs cause airway smooth muscles to relax by blocking stimulation from cholinergic nerves. Ipratropium is the most widely prescribed short acting anticholinergic drug. Like short-acting β2 agonists, short-acting anticholinergics provide rapid relief of COPD symptoms and a combination of the two is commonly used for a greater bronchodilator effect. Tiotropium is the most commonly prescribed long-acting anticholinergic drug in COPD. It is has more specificity for M3 muscarinic receptors so may have less side-effects than other anticholinergic drugs. Regular use is associated with improvements in airflow, exercise capacity, quality of life and possibly a longer life. [35] [36]
Corticosteroids
Corticosteroids act to reduce the inflammation in the airways, in theory reducing lung damage and airway narrowing caused by inflammation.[37] Unlike bronchodilators, they do not act directly on the airway smooth muscle and do not provide immediate relief of symptoms. Some of the more common corticosteroids in use are prednisone, fluticasone, budesonide, mometasone, and beclomethasone. Corticosteroids are used in tablet or inhaled form to treat and prevent acute exacerbations of COPD. Inhaled corticosteroids have not been shown to be of benefit for people with mild COPD however they are beneficial for those with either moderate of severe COPD.[38] Most people with COPD who use inhaled corticosteroids also use a long-acting bronchodilator so inhaled corticosteroids are often combined with a LABA in the same inhaler.[citation needed]
Other medication
Theophylline is a bronchodilator and phosphodiesterase inhibitor that in high doses can reduce symptoms for some people who have COPD. More often, side effects such as nausea and stimulation of the heart limit its use.[1] In lower doses, it may slightly reduce the number of COPD exacerbations.[39] The investigative phosphodiesterase-4 antagonists, roflumilast and cilomilast have completed Phase-2 clinical trials. Tumor necrosis factor antagonists such as infliximab suppress the immune system and reduce inflammation. Infliximab has been trialled in COPD but there was no evidence of benefit with the possibility of harm.[40]
Supplemental oxygen
Oxygen can be delivered in different forms: in large containers, in smaller containers with liquid oxygen, or with the use of a oxygen concentrator (shown here) which derives oxygen from room air. The latter two options improve mobility of people requiring long-term oxygen therapy.
Supplemental oxygen can be given to people with COPD who have low oxygen levels in the body. Oxygen is provided from an oxygen cylinder or an oxygen concentrator and delivered to a person through tubing via a nasal cannula or oxygen mask. Supplemental oxygen does not greatly improve shortness of breath but can allow people with COPD and low oxygen levels to do more exercise and household activity. Long-term oxygen therapy for at least 16 hours a day can improve the quality of life and survival for people with COPD and arterial hypoxemia or with complications of hypoxemia such as pulmonary hypertension, cor pulmonale, or secondary erythrocytosis.[41] High concentrations of supplemental oxygen can lead to the accumulation of carbon dioxide and respiratory acidosis for some people with severe COPD; lower oxygen flow rates are generally safer for these individuals.
Pulmonary rehabilitation
Pulmonary rehabilitation is a program of exercise, disease management and counselling coordinated to benefit the individual.[42] Pulmonary rehabilitation has been shown to improve shortness of breath and exercise capacity. It has also been shown to improve the sense of control a patient has over their disease as well as their emotions.[43]
Nutrition
Being either underweight or overweight can affect the symptoms, degree of disability and prognosis of COPD. People with COPD who are underweight can improve their breathing muscle strength by increasing their calorie intake.[1] When combined with regular exercise or a pulmonary rehabilitation programme, this can lead to improvements in COPD symptoms.
Surgery
Surgery is sometimes helpful for COPD in selected cases. A bullectomy is the surgical removal of a bulla, a large air-filled space that can squash the surrounding, more normal lung. Lung volume reduction surgery is similar; parts of the lung that are particularly damaged by emphysema are removed allowing the remaining, relatively good lung to expand and work better. Lung transplantation is sometimes performed for severe COPD, particularly in younger individuals.
Management of acute exacerbations
Acute exacerbations can be partially prevented. Infections are responsible for approximately half of COPD exacerbations, some of which can be prevented by vaccination against pathogens such as influenza and Streptococcus pneumoniae. Regular medication use can prevent some COPD exacerbations; LABAs, long-acting anticholinergics, inhaled corticosteroids and low-dose theophylline have all been shown to reduce the frequency of COPD exacerbations.[34] [35][44][39] The symptoms of acute exacerbations are treated using short-acting bronchodilators. A course of corticosteroids, usually in tablet or intravenous rather than inhaled form, can speed up recovery. [1] Antibiotics are often used but will only help if the exacerbation is due to an infection.[45] Antibiotics are indicated when a patient notes sputum changes, has an elevated white count, or is febrile. Severe exacerbations can require hospital care where treatments such as oxygen and mechanical ventilation may be required.[46]
Prognosis
COPD usually gradually gets worse over time and can lead to death. The rate at which it gets worse varies between individuals. The factors that predict a poorer prognosis are: [1]
• Severe airflow obstruction (low FEV1)
• Poor exercise capacity
• Shortness of breath
• Significantly underweight or overweight
• Complications like respiratory failure or cor pulmonale
• Continued smoking
• Frequent acute exacerbations
History
COPD has probably always existed but has been called by different names in the past. Bonet described a condition of “voluminous lungs” in 1679. In 1769,Giovanni Morgagni described 19 cases where the lungs were “turgid” particularly from air.[47] The first description and illustration of the enlarged airspaces in emphysema was provided by Ruysh in 1721."History of pathologic descriptions of COPD" (PDF). http://www.mhprofessional.com/downloads/products/0071457399/0071457399_chap40.pdf. Matthew Baillie illustrated an emphysematous lung in 1789 and described the destructive character of the condition.[47] Badham used the word "catarrh" to describe the cough and mucus hypersecretion of chronic bronchitis in 1814. He recognised that chronic bronchitis was a disabling disorder.
René Laennec, the physician who invented the stethoscope, used the term emphysema in his 1837 book A treatise on the diseases of the chest and of mediate auscultation to describe lungs that did not collapse when he opened the chest during an autopsy. He noted that they did not collapse as usual because they were full of air and the airways were filled with mucus.[47]
John Hutchinson invented the spirometer in 1842 that allowed the measurement of vital airflow.[48] Tiffeneau in 1947 and Gaensler in 1950 and 1951 described the principles of measuring airflow.capacity of the lungs however his spirometer could only measure volume, not
The terms chronic bronchitis and emphysema were formally defined at the CIBA guest symposium of physicians in 1959. The term COPD was first used by William Briscoe in 1965 and has gradually overtaken other terms to become established today as the preferred name of this disease
Smoke
The Harmful Effects of Cigarette Smoking on the Cardiovascular System
Short Term Long Term
Cigarette smoking damages the body slowly and greatly, and one of the areas where smoking causes the most problems is in the cardiovascular system. The cardiovascular system is composed of your heart, lungs, and circulatory system. Smoking creates a lot of problems here because you constantly use your cardiovascular system.
You use it simply by breathing, and when you smoke, all the tar from the cigarettes slowly clog up the aviolie in your lungs. Aviolie are the areas where oxygen is defused into the blood stream and the more of these that are clogged the harder it is for oxygen to circulate to the body. This will lead to wheezing and shortness of breath.
Cigarette smoking has many irreversible health effects associated with it, which can be short or long term on your respiratory system and heart. Smoking directly damages your respiratory system. For example, a person who smokes one pack of cigarettes a day for one year is doing the same as smearing one cup of tar on his/her lungs. Tobacco smoke contains 4000 different chemicals some of which are Carbon Monoxide, Ammonia, Hydrogen Cyanide, and Lead. These substances combined can seriously damage the body and can even be fatal.
SHORT TERM:
Some short term effects of this include bad breath, coughing, wheezing and sputum production which may lead to respiratory infection which can cause permanent lung tissue damage. This damage can be reduced if you quit smoking but it can never be reversed.
The effects of cigarette smoke are just as bad for non-smokers as for smokers. The smoke at the end of a burning cigarette is more harmful to the non-smoker than the smoke inhaled by the smoker because the particles are smaller and can go deeper into the lung and can be more damaging. The non-smoker can develop the same health complications as the smoker.
Smoking as an adolescent is a very bad idea. It will cause retardation in the development of a teenager’s lung and their lungs will never fully attain normal development and function. This may cause an earlier decline in lung functions creating a deceased lung reserve which may leave them unable to walk or run for extended periods of time.
Almost 75% of long-term smokers showed changes in their DNA patterns which led to pancreas lesions which is experienced by only 3% of non-smokers.
Smoking is the primary factor in many diseases. It accounts for 80-90% of chronic obstructive pulmonary diseases such as chronic mucus secretion, emphysema, and chronic airflow along with bronchitis. Smokers are more susceptible to bacterial infections, nose and throat inflammations and respiratory infections.
LONG TERM:
The heart and circulatory system are also greatly effected by cigarette smoking. Smoking is one of the leading factors of heart disease and if continued for a long period of time could lead to death. Premature coronary heart disease could lead to sudden death. Atherosclerosis is caused by smoking. This is a build up of fatty deposits on the inside of the carotid artery which can lead to blood vessel diseases and narrowing and stiffening of blood vessels which could rupture easier. This increases cholesterol and clogging of arteries which, in turn, will lead to poor circulation to the extremities of the body and high blood pressure and a higher heart rate. At least 30% of people suffering with heart disease will end up dying. The circulatory system may be repaired with surgery but if a person’s circulatory system is so far gone, it is only a matter of time before they die. Heart disease may be accompanied by chest spasms, which is a symptom of a heart attack which may lead to death.
The most common disease associated with smoking is cancer of the lung.
Smoking also causes cancer of the lips, tongue, salivary glands, mouth, larynx, esophagus, and middle and lower pharynx, bladder. It is also linked to cancers of the renal pelvis, uterine cervix and pancreas. All of these cancers are irreversible and you will die a long, painful death. Smoking is the leading cause of death in Canada.
There should be no questions about the dangers of cigarette smoking and the effects it has on the cardiovascular system. In conclusion, the previous information about these effects has all been scientifically proven to be linked to cigarette smoking.
Hot Links
Constituents of Tobacco Smoke
Harmful Effects of Smoking
Selected Causes of Preventable Deaths, Canada 1989
Short Term Long Term
Cigarette smoking damages the body slowly and greatly, and one of the areas where smoking causes the most problems is in the cardiovascular system. The cardiovascular system is composed of your heart, lungs, and circulatory system. Smoking creates a lot of problems here because you constantly use your cardiovascular system.
You use it simply by breathing, and when you smoke, all the tar from the cigarettes slowly clog up the aviolie in your lungs. Aviolie are the areas where oxygen is defused into the blood stream and the more of these that are clogged the harder it is for oxygen to circulate to the body. This will lead to wheezing and shortness of breath.
Cigarette smoking has many irreversible health effects associated with it, which can be short or long term on your respiratory system and heart. Smoking directly damages your respiratory system. For example, a person who smokes one pack of cigarettes a day for one year is doing the same as smearing one cup of tar on his/her lungs. Tobacco smoke contains 4000 different chemicals some of which are Carbon Monoxide, Ammonia, Hydrogen Cyanide, and Lead. These substances combined can seriously damage the body and can even be fatal.
SHORT TERM:
Some short term effects of this include bad breath, coughing, wheezing and sputum production which may lead to respiratory infection which can cause permanent lung tissue damage. This damage can be reduced if you quit smoking but it can never be reversed.
The effects of cigarette smoke are just as bad for non-smokers as for smokers. The smoke at the end of a burning cigarette is more harmful to the non-smoker than the smoke inhaled by the smoker because the particles are smaller and can go deeper into the lung and can be more damaging. The non-smoker can develop the same health complications as the smoker.
Smoking as an adolescent is a very bad idea. It will cause retardation in the development of a teenager’s lung and their lungs will never fully attain normal development and function. This may cause an earlier decline in lung functions creating a deceased lung reserve which may leave them unable to walk or run for extended periods of time.
Almost 75% of long-term smokers showed changes in their DNA patterns which led to pancreas lesions which is experienced by only 3% of non-smokers.
Smoking is the primary factor in many diseases. It accounts for 80-90% of chronic obstructive pulmonary diseases such as chronic mucus secretion, emphysema, and chronic airflow along with bronchitis. Smokers are more susceptible to bacterial infections, nose and throat inflammations and respiratory infections.
LONG TERM:
The heart and circulatory system are also greatly effected by cigarette smoking. Smoking is one of the leading factors of heart disease and if continued for a long period of time could lead to death. Premature coronary heart disease could lead to sudden death. Atherosclerosis is caused by smoking. This is a build up of fatty deposits on the inside of the carotid artery which can lead to blood vessel diseases and narrowing and stiffening of blood vessels which could rupture easier. This increases cholesterol and clogging of arteries which, in turn, will lead to poor circulation to the extremities of the body and high blood pressure and a higher heart rate. At least 30% of people suffering with heart disease will end up dying. The circulatory system may be repaired with surgery but if a person’s circulatory system is so far gone, it is only a matter of time before they die. Heart disease may be accompanied by chest spasms, which is a symptom of a heart attack which may lead to death.
The most common disease associated with smoking is cancer of the lung.
Smoking also causes cancer of the lips, tongue, salivary glands, mouth, larynx, esophagus, and middle and lower pharynx, bladder. It is also linked to cancers of the renal pelvis, uterine cervix and pancreas. All of these cancers are irreversible and you will die a long, painful death. Smoking is the leading cause of death in Canada.
There should be no questions about the dangers of cigarette smoking and the effects it has on the cardiovascular system. In conclusion, the previous information about these effects has all been scientifically proven to be linked to cigarette smoking.
Hot Links
Constituents of Tobacco Smoke
Harmful Effects of Smoking
Selected Causes of Preventable Deaths, Canada 1989
GIT Disease
Crohn's disease
From Wikipedia, the free encyclopedia
Jump to: navigation, search
Crohn's disease
Classification and external resources
The three most common sites of intestinal involvement in Crohn's disease are
ileal, ileocolic and colonic.[1]
ICD-10
K50.
ICD-9
555
OMIM
266600
DiseasesDB
3178
MedlinePlus
000249
eMedicine
med/477 ped/507 radio/197
MeSH
D003424
Crohn's disease (also known as granulomatous colitis and regional enteritis) is an inflammatory disease which may affect any part of the gastrointestinal tract from mouth to anus, causing a wide variety of symptoms. It primarily causes abdominal pain, diarrhea (which may be bloody), vomiting, or weight loss,[1][2][3] but may also cause complications outside of the gastrointestinal tract such as skin rashes, arthritis and inflammation of the eye.[1]
Crohn's disease is an autoimmune disease, caused by the immune system attacking the gastrointestinal tract and producing inflammation in the gastrointestinal tract; it is classified as a type of inflammatory bowel disease. There appears to be a genetic link to Crohn's disease, with the highest risk occurring in individuals with siblings who have the disease. Males and females are equally affected. Smokers are three times more likely to develop Crohn's disease. [4] Crohn's disease affects between 400,000 and 600,000 people in North America.[5] Prevalence estimates for Northern Europe have ranged from 27–48 per 100,000.[6] Crohn's disease tends to present initially in the teens and twenties, with another peak incidence in the fifties to seventies, although the disease can occur at any age.[1][7]
There is no known drug or surgical cure for Crohn's disease;[8] treatment options are restricted to controlling symptoms, maintaining remission and preventing relapse.
The disease was independently described in 1904 by Polish surgeon Antoni Leśniowski and in 1932 by American gastroenterologist Burrill Bernard Crohn, for whom the disease was named. Crohn, along with two colleagues, described a series of patients with inflammation of the terminal ileum, the area most commonly affected by the illness.[9] For this reason, the disease has also been called regional ileitis[9] or regional enteritis.
Classification
Distribution of gastrointestinal Crohn's disease. Based on data from American Gastroenterological Association.
Crohn's disease is generally classified as an autoimmune disease. It invariably affects the gastrointestinal tract, and most gastroenterologists categorize the presenting disease by the affected areas. Ileocolic Crohn's disease, which affects both the ileum (the last part of the small intestine that connects to the large intestine) and the large intestine, accounts for fifty percent of cases. Crohn's ileitis, affecting the ileum only, accounts for thirty percent of cases, and Crohn's colitis, affecting the large intestine, accounts for the remaining twenty percent of cases and may be particularly difficult to distinguish from ulcerative colitis. The disease can attack any part of the digestive tract, from mouth to anus. However, individuals affected by the disease rarely fall outside these three classifications, being affected in other parts of the gastrointestinal tract such as the stomach and esophagus.[1] Crohn's disease may also be categorized by the behavior of disease as it progresses. This was formalized in the Vienna classification of Crohn's disease.[10] There are three categories of disease presentation in Crohn's disease: stricturing, penetrating, and inflammatory. Stricturing disease causes narrowing of the bowel which may lead to bowel obstruction or changes in the caliber of the feces. Penetrating disease creates abnormal passageways (fistulae) between the bowel and other structures such as the skin. Inflammatory disease (or non-stricturing, non-penetrating disease) causes inflammation without causing strictures or fistulae.[10][11]
Symptoms
Endoscopy image of colon showing serpiginous ulcer, a classic finding in Crohn's disease
Many people with Crohn's disease have symptoms for years prior to the diagnosis.[12] The usual onset is between 15 and 30 years of age but can occur at any age.[13] Because of the 'patchy' nature of the gastrointestinal disease and the depth of tissue involvement, initial symptoms can be more vague than with ulcerative colitis. People with Crohn's disease will go through periods of flare-ups and remission.
Gastrointestinal symptoms
Abdominal pain may be the initial symptom of Crohn's disease. It is often accompanied by diarrhea, which may or may not be bloody, though diarrhea is not uncommon especially in those who have had surgery. People who have had surgery or multiple surgeries often end up with short bowel syndrome of the gastrointestinal tract. The nature of the diarrhea in Crohn's disease depends on the part of the small intestine or colon that is involved. Ileitis typically results in large-volume watery feces. Colitis may result in a smaller volume of feces of higher frequency. Fecal consistency may range from solid to watery. In severe cases, an individual may have more than 20 bowel movements per day and may need to awaken at night to defecate.[1][7][14][15] Visible bleeding in the feces is less common in Crohn's disease than in ulcerative colitis, but may be seen in the setting of Crohn's colitis.[1] Bloody bowel movements are typically intermittent, and may be bright or dark red in colour. In the setting of severe Crohn's colitis, bleeding may be copious.[7] Flatulence and bloating may also add to the intestinal discomfort.[7]
Symptoms caused by intestinal stenosis are also common in Crohn's disease. Abdominal pain is often most severe in areas of the bowel with stenoses. In the setting of severe stenosis, vomiting and nausea may indicate the beginnings of small bowel obstruction.[7] Crohn's disease may also be associated with primary sclerosing cholangitis, a type of inflammation of the bile ducts.[citation needed]
Perianal discomfort may also be prominent in Crohn's disease. Itchiness or pain around the anus may be suggestive of inflammation, fistulization or abscess around the anal area[1] or anal fissure. Perianal skin tags are also common in Crohn's disease.[16] Fecal incontinence may accompany peri-anal Crohn's disease. At the opposite end of the gastrointestinal tract, the mouth may be affected by non-healing sores (aphthous ulcers). Rarely, the esophagus, and stomach may be involved in Crohn's disease. These can cause symptoms including difficulty swallowing (odynophagia), upper abdominal pain, and vomiting.[17]
Systemic symptoms
Crohn's disease, like many other chronic, inflammatory diseases, can cause a variety of systemic symptoms.[1] Among children, growth failure is common. Many children are first diagnosed with Crohn's disease based on inability to maintain growth.[18] As Crohn's disease may manifest at the time of the growth spurt in puberty, up to 30% of children with Crohn's disease may have retardation of growth.[19] Fever may also be present, though fevers greater than 38.5 ˚C (101.3 ˚F) are uncommon unless there is a complication such as an abscess[1] Among older individuals, Crohn's disease may manifest as weight loss. This is usually related to decreased food intake, since individuals with intestinal symptoms from Crohn's disease often feel better when they do not eat and might lose their appetite.[18] People with extensive small intestine disease may also have malabsorption of carbohydrates or lipids, which can further exacerbate weight loss.[20]
Extraintestinal symptoms
Erythema nodosum
In addition to systemic and gastrointestinal involvement, Crohn's disease can affect many other organ systems.[21] Inflammation of the interior portion of the eye, known as uveitis, can cause eye pain, especially when exposed to light (photophobia). Inflammation may also involve the white part of the eye (sclera), a condition called episcleritis. Both episcleritis and uveitis can lead to loss of vision if untreated.
Crohn's disease is associated with a type of rheumatologic disease known as seronegative spondyloarthropathy. This group of diseases is characterized by inflammation of one or more joints (arthritis) or muscle insertions (enthesitis). The arthritis can affect larger joints such as the knee or shoulder or may exclusively involve the small joints of the hand and feet. The arthritis may also involve the spine, leading to ankylosing spondylitis if the entire spine is involved or simply sacroiliitis if only the lower spine is involved. The symptoms of arthritis include painful, warm, swollen, stiff joints and loss of joint mobility or function.[citation needed]
Crohn's disease may also involve the skin, blood, and endocrine system. One type of skin manifestation, erythema nodosum, presents as red nodules usually appearing on the shins. Erythema nodosum is due to inflammation of the underlying subcutaneous tissue and is characterized by septal panniculitis. Another skin lesion, pyoderma gangrenosum, is typically a painful ulcerating nodule. Crohn's disease also increases the risk of blood clots; painful swelling of the lower legs can be a sign of deep venous thrombosis, while difficulty breathing may be a result of pulmonary embolism. Autoimmune hemolytic anemia, a condition in which the immune system attacks the red blood cells, is also more common in Crohn's disease and may cause fatigue, pallor, and other symptoms common in anemia. Clubbing, a deformity of the ends of the fingers, may also be a result of Crohn's disease. Finally, Crohn's disease may cause osteoporosis, or thinning of the bones. Individuals with osteoporosis are at increased risk of bone fractures.[6]
Crohn's disease can also cause neurological complications (reportedly in up to 15% of patients).[22] The most common of these are seizures, stroke, myopathy, peripheral neuropathy, headache and depression.[22]
Crohn's patients often also have issues with Small bowel bacterial overgrowth syndrome, which has similar symptoms.[23]
Complications
Endoscopic image of colon cancer identified in the sigmoid colon on screening colonoscopy for Crohn's disease.
Crohn's disease can lead to several mechanical complications within the intestines, including obstruction, fistulae, and abscesses. Obstruction typically occurs from strictures or adhesions which narrow the lumen, blocking the passage of the intestinal contents. Fistulae can develop between two loops of bowel, between the bowel and bladder, between the bowel and vagina, and between the bowel and skin. Abscesses are walled off collections of infection, which can occur in the abdomen or in the perianal area in Crohn's disease sufferers.
Crohn's disease also increases the risk of cancer in the area of inflammation. For example, individuals with Crohn's disease involving the small bowel are at higher risk for small intestinal cancer. Similarly, people with Crohn's colitis have a relative risk of 5.6 for developing colon cancer.[24] Screening for colon cancer with colonoscopy is recommended for anyone who has had Crohn's colitis for eight years, or more.[25] Some study suggest that there is a role for chimioprotection in the prevention of colorectal cancer in Crohn's involving the colon; two agents have been suggested, folate and mesalamine preparations.[26]
Individuals with Crohn's disease are at risk of malnutrition for many reasons, including decreased food intake and malabsorption. The risk increases following resection of the small bowel. Such individuals may require oral supplements to increase their caloric intake, or in severe cases, total parenteral nutrition (TPN). Most people with moderate or severe Crohn's disease are referred to a dietitian for assistance in nutrition.[27]
Crohn's disease can cause significant complications including bowel obstruction, abscesses, free perforation and hemorrhage.[28]
Crohn's disease can be problematic during pregnancy, and some medications can cause adverse outcomes for the fetus or mother. Consultation with an obstetrician and gastroenterologist about Crohn's disease and all medications allows preventative measures to be taken. In some cases, remission can occur during pregnancy. Certain medications can also impact sperm count or may otherwise adversely affect a man's ability to conceive.[29]
Cause
The exact cause of Crohn's disease is unknown. Environmental and genetic factors have been invoked in the pathogenesis of the disease.
Genetics
Schematic of NOD2 CARD15 gene, which is associated with certain disease patterns in Crohn's disease.
Research has indicated that Crohn's disease has a strong genetic link.[30] The disease runs in families and those with a sibling with the disease are 30 times more likely to develop it than the normal population. Ethnic background is also a risk factor.
Mutations in the CARD15 gene (also known as the NOD2 gene) are associated with Crohn's disease[31] and with susceptibility to certain phenotypes of disease location and activity.[32] In earlier studies, only two genes were linked to Crohn's, but scientists now believe there are over eight genes that show genetics play a role in the disease, either directly through causation or indirectly as with a mediator variable. Anomalies in the XBP1 gene have recently been identified as a factor, pointing towards a role for the unfolded protein response pathway of the endoplasmatic reticulum in inflammatory bowel diseases.[33][34]
Environmental factors
A handful of cases of Crohn's disease cases were reported at the turn of the 20th century, but since then, the disease has continued to increase in prevalence dramatically. Some argue that this increase has been the result of a genetic shift in the population caused by conditions favoring individuals carrying the genes linked with the disease. These conditions could be a lower infant mortality rate or better health care in the nations that have the highest incidence of disease (industrialized nations). Another explanation is that modern industrial practices have given rise to increased disease prevalence via infectious diseases. A common recurrent theory is that a specific species of Mycobacterium, Mycobacterium avium subspecies paratuberculosis, is responsible for both Johne's disease and Crohn's disease via modern industrial farming practices.[citation needed]
Smoking has been shown to increase the risk of the return of active disease, or "flares".[35] The introduction of hormonal contraception in the United States in the 1960s is linked with a dramatic increase in the incidence rate of Crohn's disease. Although a causal linkage has not been effectively shown, there remain fears that these drugs work on the digestive system in similar ways to smoking.[36]
Some scientific studies and court rulings have posited that Accutane is a probable cause of IBD, a group of diseases including Crohn's Disease and Ulcerative colitis, in some individuals. Three cases in the United States have gone to trial thus far, with all three resulting in multi-million dollar judgments against the makers of isotretinoin; there are an additional 425 cases pending. [37] [38] [39] [40] [41]
Immune system
Abnormalities in the immune system have often been invoked as being causes of Crohn's disease. Crohn's disease is thought to be an autoimmune disease, with inflammation stimulated by an over-active Th1 cytokine response.[42] However, more recent evidence has shown that Th17 is of greater importance in the disease.[43] The most recent gene to be implicated in Crohn's disease is ATG16L1, which may reduce the effectiveness of autophagy, and hinder the body's ability to attack invasive bacteria.[44]
Contrary to the prevailing view that Crohn's disease is a primary T cell autoimmune disorder, there is an increasing body of evidence in favour of the hypothesis that Crohn's disease results from an impaired innate immunity.[45] The immunodeficiency, which has been shown to be due to (at least in part) impaired cytokine secretion by macrophages,[46] is thought to lead to a sustained microbial-induced inflammatory response, particularly in the colon where the bacterial load is especially high. [47]
Microbes
A variety of pathogenic bacteria were initially suspected of being causative agents of Crohn's disease and many groups still suspect Mycobacterium avium subspecies paratuberculosis.[48] However, the consensus of many health care professionals for several years has been that a variety of microorganisms are simply taking advantage of their host's weakened mucosal layer and inability to clear bacteria from the intestinal walls, both symptoms of the disease.[49] Some studies have linked Mycobacterium avium subsp. paratuberculosis to Crohn's disease, in part because it causes a very similar disease, Johne's disease, in cattle.[50] The mannose bearing antigens, mannins, from yeast may also elicit pathogenic anti saccharomyces cerevisiae antibodies.[51] Studies have linked specific strains of enteroadherent E. coli to the disease. Still this relationship and evidence of contributions by other species remains controversial.[52][53][54][55] And more recently a relationship between MAP and E. coli has been uncovered. Scientists at the university of Liverpool have discovered that "Mycobacteria release a complex molecule containing a sugar, called mannose. This molecule prevents a type of white blood cells, called macrophages, from killing internalized E.Coli.”[56]
Some studies have suggested that Crohn's disease, ulcerative colitis and irritable bowel syndrome have the same underlying cause. Biopsy samples taken from the colons of all three patient groups were found to produce elevated levels of a serine protease. [57] Experimental introduction of the serine protease into mice has been found to produce widespread pain associated with irritable bowel syndrome as well as colitis, which is associated with all three diseases.[58] The authors of that study were unable to identify the source of the protease, but a separate review noted that regional and temporal variations in those illnesses follow those associated with infection with a poorly understood protozoan, Blastocystis. [59]
A study in 2003 put forth the "cold-chain" hypothesis, that psychrotrophic bacteria such as Yersinia spp and Listeria spp contribute to the disease. A statistical correlation was found between the advent of the use of refrigeration in the United States and various parts of Europe and the rise of the disease. [60] [61] Later studies have provided support for this hypothesis[62].
Pathophysiology
Section of colectomy showing transmural inflammation
At the time of colonoscopy, biopsies of the colon are often taken in order to confirm the diagnosis. There are certain characteristic features of the pathology seen that point toward Crohn's disease. Crohn's disease shows a transmural pattern of inflammation, meaning that the inflammation may span the entire depth of the intestinal wall.[1] Grossly, ulceration is an outcome seen in highly active disease. There is usually an abrupt transition between unaffected tissue and the ulcer. Under a microscope, biopsies of the affected colon may show mucosal inflammation. This inflammation is characterized by focal infiltration of neutrophils, a type of inflammatory cell, into the epithelium. This typically occurs in the area overlying lymphoid aggregates. These neutrophils, along with mononuclear cells, may infiltrate into the crypts leading to inflammation (crypititis) or abscess (crypt abscess). Granulomas, aggregates of macrophage derivatives known as giant cells, are found in 50% of cases and are most specific for Crohn's disease. The granulomas of Crohn's disease do not show "caseation", a cheese-like appearance on microscopic examination that is characteristic of granulomas associated with infections such as tuberculosis. Biopsies may also show chronic mucosal damage as evidenced by blunting of the intestinal villi, atypical branching of the crypts, and change in the tissue type (metaplasia). One example of such metaplasia, Paneth cell metaplasia, involves development of Paneth cells (typically found in the small intestine) in other parts of the gastrointestinal system.[63]
According to the volume 1 of the 10th Edition of Brunner and Suddarth’s Textbook of Medical-Surgical Nursing Regional enteritis is a subacute and chronic inflammation that extends through the layers (ie, transmural lesion) of the bowel wall from the intestinal mucosa. It is characterized by periods of remissions and exacerbations. The disease process begins with edema and thickening of the mucosa. Ulcers begin to appear on the inflamed mucosa. These lesions are not in continuous contact with one another and are separated by normal tissue. Fistulas, fissures, and abscesses form as the inflammation extends into the peritoneum. Granulomas occur in one half of patients. In advanced cases, the intestinal mucosa has a cobblestone appearance. As the disease advances, the bowel wall thickens and becomes fibrotic, and the intestinal lumen narrows. Diseased bowel loops sometimes adhere to other loops surrounding them.
Diagnosis
Endoscopic image of Crohn's colitis showing deep ulceration
CT scan showing Crohn's disease in the fundus of the stomach
Crohn's disease can mimic ulcerative colitis on endoscopy. This endoscopic image is of Crohn's colitis showing diffuse loss of mucosal architecture, friability of mucosa in sigmoid colon and exudate on wall, all of which can be found with ulcerative colitis.
The diagnosis of Crohn's disease can sometimes be challenging,[12] and a number of tests are often required to assist the physician in making the diagnosis.[7] Even with a full battery of tests it may not be possible to diagnose Crohn's with complete certainty; a colonoscopy is approximately 70% effective in diagnosing the disease with further being less effective. Disease in the small bowel is particularly difficult to diagnose as a traditional colonoscopy only allows access to the colon and lower portions of the small intestines; introduction of the Capsule endoscopy[64] aids in endoscopic diagnosis.
Endoscopy
A colonoscopy is the best test for making the diagnosis of Crohn's disease as it allows direct visualization of the colon and the terminal ileum, identifying the pattern of disease involvement. Occasionally, the colonoscope can travel past the terminal ileum but it varies from patient to patient. During the procedure, the gastroenterologist can also perform a biopsy, taking small samples of tissue for laboratory analysis which may help confirm a diagnosis. As 30% of Crohn's disease involves only the ileum,[1] cannulation of the terminal ileum is required in making the diagnosis. Finding a patchy distribution of disease, with involvement of the colon or ileum but not the rectum, is suggestive of Crohn's disease, as are other endoscopic stigmata.[65]The utility of capsule endoscopy for this, however, is still uncertain.[66]
Radiologic tests
A small bowel follow-through may suggest the diagnosis of Crohn's disease and is useful when the disease involves only the small intestine. Because colonoscopy and gastroscopy allow direct visualization of only the terminal ileum and beginning of the duodenum, they cannot be used to evaluate the remainder of the small intestine. As a result, a barium follow-through x-ray, wherein barium sulfate suspension is ingested and fluoroscopic images of the bowel are taken over time, is useful for looking for inflammation and narrowing of the small bowel.[65][67] Barium enemas, in which barium is inserted into the rectum and fluoroscopy used to image the bowel, are rarely used in the work-up of Crohn's disease due to the advent of colonoscopy. They remain useful for identifying anatomical abnormalities when strictures of the colon are too small for a colonoscope to pass through, or in the detection of colonic fistulae.[68]
CT and MRI scans are useful for evaluating the small bowel with enteroclysis protocols.[69]They are additionally useful for looking for intra-abdominal complications of Crohn's disease such as abscesses, small bowel obstruction, or fistulae.[70] Magnetic resonance imaging (MRI) are another option for imaging the small bowel as well as looking for complications, though it is more expensive and less readily available[71]
Blood tests
A complete blood count may reveal anemia, which may be caused either by blood loss or vitamin B12 deficiency. The latter may be seen with ileitis because vitamin B12 is absorbed in the ileum.[72] Erythrocyte sedimentation rate, or ESR, and C-reactive protein measurements can also be useful to gauge the degree of inflammation.[73] It is also true in patient with ilectomy done in response to the complication. Another cause of anaemia is anaemia of chronic disease, characterized by its microcytic and hypochromic anaemia. There can be various reasons for anaemia, including medication used in treatment of inflammatory bowel disease like azathioprine which can lead to cytopenia and sulfasalazine which can also result in folate malabsorption, etc. Testing for anti-Saccharomyces cerevisiae antibodies (ASCA) and anti-neutrophil cytoplasmic antibodies (ANCA) has been evaluated to identify inflammatory diseases of the intestine[74] and to differentiate Crohn's disease from ulcerative colitis.[75]
Feces
Calprotectin is a protein found in neutrophil cytosol. Neutrophils are integral to the inflammatory process caused by Crohn's disease. Faecal Calprotectin is a protein measured in a patient's feces. The fecal concentration correlates well with inflammation and disease activity. Normal fecal calprotectin level in a patient with active gastrointestinal symptoms excludes inflammatory bowel disease as a likely diagnosis and in many cases negates the need for colonoscopy or radio labelled white cell scanning[76].
Comparison with ulcerative colitis
The most common disease that mimics the symptoms of Crohn's disease is ulcerative colitis, as both are inflammatory bowel diseases that can affect the colon with similar symptoms. It is important to differentiate these diseases, since the course of the diseases and treatments may be different. In some cases, however, it may not be possible to tell the difference, in which case the disease is classified as indeterminate colitis.[14][1][7]
Comparisons of various factors in Crohn's disease and ulcerative colitis
Crohn's disease Ulcerative colitis
Terminal ileum involvement Commonly Seldom
Colon involvement Usually Always
Rectum involvement Seldom Usually[77]
Involvement around the anus
Common[78]
Seldom
Bile duct involvement No increase in rate of primary sclerosing cholangitis
Higher rate[79]
Distribution of Disease Patchy areas of inflammation (Skip lesions) Continuous area of inflammation[77]
Endoscopy Deep geographic and serpiginous (snake-like) ulcers
Continuous ulcer
Depth of inflammation May be transmural, deep into tissues[78][1]
Shallow, mucosal
Fistulae
Common[78]
Seldom
Stenosis
Common Seldom
Autoimmune disease
Widely regarded as an autoimmune disease No consensus
Cytokine response
Associated with Th17 [43]
Vaguely associated with Th2
Granulomas on biopsy Can have granulomas[78]
Granulomas not seen[77]
Surgical cure Often returns following removal of affected part Usually cured by removal of colon
Smoking
Higher risk for smokers Lower risk for smokers[77]
Treatment
Main articles: Treatment of Crohn's disease and Biological therapy for inflammatory bowel disease
Currently there is no cure for Crohn's disease and remission may not be possible or prolonged if achieved, although in recent studies low dose naltroxen has shown improvement in Crohn's disease.;[80] in cases where remission is possible, relapse can be prevented and symptoms controlled with medication, lifestyle changes and in some cases, surgery. Adequately controlled, Crohn's disease may not significantly restrict daily living.[81] Treatment for Crohn's disease is only when symptoms are active and involve first treating the acute problem, then maintaining remission.
Medication
Acute treatment uses medications to treat any infection (normally antibiotics) and to reduce inflammation (normally aminosalicylate anti-inflammatory drugs and corticosteroids). When symptoms are in remission, treatment enters maintenance with a goal of avoiding the recurrence of symptoms. Prolonged use of corticosteroids has significant side-effects; as a result they are generally not used for long-term treatment. Alternatives include aminosalicylates alone, though only a minority are able to maintain the treatment, and many require immunosuppressive drugs.[78]
Medications used to treat the symptoms of Crohn's disease include 5-aminosalicylic acid (5-ASA) formulations, prednisone, immunomodulators such as azathioprine, mercaptopurine, methotrexate, infliximab, adalimumab[14] and natalizumab.[82][83] Hydrocortisone should be used in severe attacks of Crohn's disease.[84]
Lifestyle changes
Certain lifestyle changes can reduce symptoms, including dietary adjustments, proper hydration and smoking cessation. Eating small meals frequently instead of big meals may help with a low appetite. To manage symptoms have a balanced diet with proper portion control. Fatigue can be helped with regular excercise, a healthy diet and enough sleep. A food diary may help with identifying foods that trigger symptoms. Some patients should follow a low fiber diet to control symptoms especially if fiberous foods cause symptoms.[81]
Surgery
Crohn's cannot be cured by surgery, though it is used when partial or a full blockage of the intestine occurs. Surgery may also be required for complications such as obstructions, fistulas and/or abscesses, or if the disease does not respond to drugs. After the first surgery, Crohn's usually shows up at the site of the resection though it can appear in other locations. After a resection, scar tissue builds up which can cause strictures. A stricture is when the intestines becomes too small to allow excrement to pass through easily which can lead to a blockage. After the first resection, another resection may be necessary within five years.[85] For patients with an obstruction due to a stricture, two options for treatment are strictureplasty and resection of that portion of bowel. There is no statistical significance between strictureplasty alone versus strictureplasty and resection in cases of duodenal involvement. In these cases, re-operation rates were 31% and 27%, respectively, indicating that strictureplasty is a safe and effective treatment for selected patients with duodenal involvement.[86]
Short bowel syndrome (SBS, also short gut syndrome or simply short gut) can be caused by the surgical removal of the small intestines. It usually develops if a person has had half or more of their small intestines removed.[87] Diarrhea is the main symptom of short bowel syndrome though other symptoms may include cramping, bloating and heartburn. Short bowel syndrome is treated with changes in diet, intravenous feeding, vitamin and mineral supplements and treatment with medications. Another complication following surgery for Crohn's disease where the terminal ileum has been removed is the development of excessive watery diarrhoea. This is due to an inability to reabsorb bile acids after resection of the terminal ileum.[citation needed]
Prospective treatments
Researchers at University College London have questioned the wisdom of suppressing the immune system in Crohn's, as the problem may be an under-active rather than an over-active immune system: their study found that Crohn's patients showed an abnormally low response to an introduced infection, marked by a poor flow of blood to the wound, and the response improved when the patients were given sildenafil.[88]
Recent studies using helminthic therapy or hookworms to treat Crohn's Disease and other (non-viral) auto-immune diseases seem to yield promising results.[89]
A single, small, uncontrolled trial of patients with mild Crohn's on stable medications suggested improvement with low dose naltrexone therapy. Further studies are needed to confirm the use of this. [90]
A recent small double-blind, placebo-controlled study[91] conducted in Germany found grand wormwood to have a steroid-sparing effect with the result that 18/20 wormwood receiving patients were able to taper steroid medication while maintaining a steady improvement in CD symptoms.
Recent research in France has suggested that a shortage of the bacterium Faecalibacterium prausnitzii may cause Crohn's disease by overstimulating the immune system. The researchers said that if ongoing animal trials prove successful, human patients could benefit from a probiotic treatment with F. prausnitzii. However, it was stated "It is too early to tell whether this will lead directly to a new treatment as other probiotics have tended to produce good results in animal studies only to prove disappointing in clinical trial in Crohn's disease." [92]
Prognosis
Crohn's disease is a chronic condition for which there is currently no cure. It is characterised by periods of improvement followed by episodes when symptoms flare up. With treatment, most people achieve a healthy height and weight, and the mortality rate for the disease is low. Crohn's disease is associated with an increased risk of small bowel and colorectal carcinoma.[93]
Epidemiology
The incidence of Crohn's disease has been ascertained from population studies in Norway and the United States and is similar at 6 to 7.1:100,000.[94][95] Crohn's disease is more common in northern countries, and shows a higher preponderance in northern areas of the same country.[96] The incidence of Crohn's disease is thought to be similar in Europe but lower in Asia and Africa.[94] It also has a higher incidence in Ashkenazi Jews.[14]
Crohn's disease has a bimodal distribution in incidence as a function of age: the disease tends to strike people in their teens and 20s, and people in their 50s through to their 70s, and ages in between due to not being diagnosed with Crohn's and being diagnosed instead with irritable bowel syndrome (IBS).[1][7] It is rarely diagnosed in early childhood. It usually strikes females who are pediatric patients more severely than males.[97] However, only slightly more women than men have Crohn's disease.[98] Parents, siblings or children of people with Crohn's disease are 3 to 20 times more likely to develop the disease.[99] Twin studies show a concordance of greater than 55% for Crohn's disease.[100]
History
Inflammatory bowel diseases were described by Giovanni Battista Morgagni (1682-1771), by Polish surgeon Antoni Leśniowski in 1904 (leading to the use of the eponym "Leśniowski-Crohn disease" in Poland) and by Scottish physician T. Kennedy Dalziel in 1913.[101]
Burrill Bernard Crohn, an American gastroenterologist at New York City's Mount Sinai Hospital, described fourteen cases in 1932, and submitted them to the American Medical Association under the rubric of "Terminal ileitis: A new clinical entity". Later that year, he, along with colleagues Leon Ginzburg and Gordon Oppenheimer published the case series as "Regional ileitis: a pathologic and clinical entity".[9]
References
1. ^ a b c d e f g h i j k l m n o Baumgart DC, Sandborn WJ (2007). "Inflammatory bowel disease: clinical aspects and established and evolving therapies.". The Lancet 369 (9573): 1641–57. doi:10.1016/S0140-6736(07)60751-X. PMID 17499606.
2. ^ Mayo Clinic: Crohn's Disease
3. ^ National Digestive Diseases Information Clearinghouse
4. ^ http://www.ncbi.nlm.nih.gov/pubmed/15157822
5. ^ Loftus, E.V.; P. Schoenfeld, W. J. Sandborn (2002). "The epidemiology and natural history of Crohn's disease in population-based patient cohorts from North America: a systematic review". Alimentary Pharmacology & Therapeutics 16 (1): 51–60. doi:10.1046/j.1365-2036.2002.01140.x. PMID 11856078.
6. ^ a b Bernstein, Charles N. (2006). The Epidemiology of Inflammatory Bowel Disease in Canada: A Population-Based Study. 101. pp. 1559–68. doi:10.1111/j.1572-0241.2006.00603.x. PMID 16863561.
7. ^ a b c d e f g h Gopal, Latha; Senthil Nachimuthu (2006-05-23). "Crohn Disease". eMedicine. http://www.emedicine.com/MED/topic477.htm. Retrieved on 2006-07-02.
8. ^ Al-Ataie, M Bashar; Vishwanath N Shenoy (2005-10-04). "Ulcerative colitis". eMedicine. http://www.emedicine.com/med/topic2336.htm. Retrieved on 2006-07-02.
9. ^ a b c Crohn BB, Ginzburg L, Oppenheimer GD (2000). "Regional ileitis: a pathologic and clinical entity. 1932". Mt. Sinai J. Med. 67 (3): 263–8. PMID 10828911.
10. ^ a b Gasche C, Scholmerich J, Brynskov J, D'Haens G, Hanauer S, Irvine E, Jewell D, Rachmilewitz D, Sachar D, Sandborn W, Sutherland L (2000). "A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998". Inflamm Bowel Dis 6 (1): 8–15. PMID 10701144.
11. ^ Dubinsky MC, Fleshner PP. (2003). "Treatment of Crohn's Disease of Inflammatory, Stenotic, and Fistulizing Phenotypes". Curr Treat Options Gastroenterol 6 (3): 183–200. doi:10.1007/s11938-003-0001-1. PMID 12744819.
12. ^ a b Pimentel, Mark; Michael Chang, Evelyn J. Chow, Siamak Tabibzadeh, Viorelia Kirit-Kiriak, Stephan R. Targan, Henry C. Lin (2000). "Identification of a prodromal period in Crohn's disease but not ulcerative colitis". American Journal of Gastroenterology 95 (12): 3458–62. doi:10.1111/j.1572-0241.2000.03361.x. PMID 11151877.
13. ^ Crohn's Disease Overview
14. ^ a b c d Podolsky, Daniel K. (2002). "Inflammatory bowel disease". New England Journal of Medicine 346 (6): 417–29. doi:10.1056/NEJMra020831. PMID 12167685. http://content.nejm.org/cgi/content/extract/347/6/417. Retrieved on 2006-07-02.
15. ^ Mueller, M. H.; M. E. Kreis, M. L. Gross, H. D. Becker, T. T. Zittel & E. C. Jehle (2002). "Anorectal functional disorders in the absence of anorectal inflammation in patients with Crohn's disease". British Journal of Surgery 89 (8): 1027–31. doi:10.1046/j.1365-2168.2002.02173.x. PMID 12153630.
16. ^ Taylor B, Williams G, Hughes L, Rhodes J (1989). "The histology of anal skin tags in Crohn's disease: an aid to confirmation of the diagnosis". Int J Colorectal Dis 4 (3): 197–9. doi:10.1007/BF01649703. PMID 2769004.
17. ^ Fix, Oren K.; Jorge A. Soto, Charles W. Andrews and Francis A. Farraye (2004). "Gastroduodenal Crohn's disease". Gastrointestinel Endoscopy 60 (6): 985. doi:10.1016/S0016-5107(04)02200-X. PMID 15605018.
18. ^ a b Beattie, R.M.; N. M. Croft, J. M. Fell, N. A. Afzal and R. B. Heuschkel (2006). "Inflammatory bowel disease". Archives of Disease in Childhood 91 (5): 426–32. doi:10.1136/adc.2005.080481. PMID 16632672.
19. ^ Büller, H.A. (1997). "Problems in diagnosis of IBD in children". The Netherlands Journal of Medicine 50 (2): S8–S11. doi:10.1016/S0300-2977(96)00064-2. PMID 9050326.
20. ^ O'Keefe, S. J. (1996). "Nutrition and gastrointestinal disease". Scandinavian Journal of Gastroenterology Supplement 31 (220): 52–9. doi:10.3109/00365529609094750. PMID 8898436.
21. ^ Danese, Silvio; Stefano Semeraro, Alfredo Papa, Italia Roberto, Franco Scaldaferri, Giuseppe Fedeli, Giovanni Gasbarrini, Antonio Gasbarrini (2005). "Extraintestinal manifestations in inflammatory bowel disease". World Journal of Gastroenterology 11 (46): 7227–36. PMID 16437620. http://www.wjgnet.com/1007-9327/11/7227.asp. Retrieved on 2006-07-02.
22. ^ a b Crohn's disease. professionals.epilepsy.com. Retrieved on July 13, 2007.
23. ^ MedlinePlus Encyclopedia Small bowel bacterial overgrowth
24. ^ Ekbom A, Helmick C, Zack M, Adami H (1990). "Increased risk of large-bowel cancer in Crohn's disease with colonic involvement". Lancet 336 (8711): 357–9. doi:10.1016/0140-6736(90)91889-I. PMID 1975343.
25. ^ Collins P, Mpofu C, Watson A, Rhodes J (2006). "Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease". Cochrane Database Syst Rev: CD000279. doi:10.1002/14651858.CD000279.pub3. PMID 16625534.
26. ^ Lynne V McFarland (2008). "Colorectal cancer and dysplasia in inflammatory bowel disease". World Journal of Gastroenterology: 2665.
27. ^ Evans J, Steinhart A, Cohen Z, McLeod R (2003). "Home total parenteral nutrition: an alternative to early surgery for complicated inflammatory bowel disease". J Gastrointest Surg 7 (4): 562–6. doi:10.1016/S1091-255X(02)00132-4. PMID 12763417.
28. ^ "Complications of Crohn's Disease". https://www.livingwithcrohnsdisease.com/livingwithcrohnsdisease/crohns_disease/complications_of_crohns.html. Retrieved on 2008-01-16.
29. ^ Kaplan, C (2005-10-21). "IBD and Pregnancy: What You Need to Know". Crohn's and Colitis Foundation of America. http://www.ccfa.org/about/news/pregnancy. Retrieved on 2008-02-14.
30. ^ "Crohn's disease has strong genetic link: study". Crohn's and Colitis Foundation of America. 2007-04-16. http://www.ccfa.org/reuters/geneticlink. Retrieved on 2008-04-20.
31. ^ Ogura Y, Bonen DK, Inohara N, et al (2001). "A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease". Nature 411 (6837): 603–6. doi:10.1038/35079114. PMID 11385577.
32. ^ Cuthbert A, Fisher S, Mirza M, et al. (2002). "The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease". Gastroenterology 122 (4): 867–74. doi:10.1053/gast.2002.32415. PMID 11910337.
33. ^ Kaser et. al. (5 September 2008). "XBP1 Links ER Stress to Intestinal Inflammation and Confers Genetic Risk for Human Inflammatory Bowel Disease". Cell (Cell Press) 134: 743–756. http://www.cell.com/content/article/abstract?uid=PIIS0092867408009410.
34. ^ Clevers H (2009). "Inflammatory Bowel Disease, Stress, and the Endoplasmic Reticulum". N Engl J Med 360 (7): 726-727. http://content.nejm.org/cgi/content/full/360/7/726.
35. ^ Cosnes J (2004). "Tobacco and IBD: relevance in the understanding of disease mechanisms and clinical practice". Best Pract Res Clin Gastroenterol 18 (3): 481–96. doi:10.1016/j.bpg.2003.12.003. PMID 15157822.
36. ^ Lesko S, Kaufman D, Rosenberg L, et al. (1985). "Evidence for an increased risk of Crohn's disease in oral contraceptive users". Gastroenterology 89 (5): 1046–9. PMID 4043662.
37. ^ "Washington Post: Roche Found Liable". http://www.washingtonpost.com/wp-dyn/content/article/2007/05/29/AR2007052901946.html. Retrieved on 2008-06-21.
38. ^ Reddy D, Siegel CA, Sands BE, Kane S (2006). "Possible association between isotretinoin and inflammatory bowel disease". Am. J. Gastroenterol. 101 (7): 1569–73. doi:10.1111/j.1572-0241.2006.00632.x. PMID 16863562.
39. ^ Borobio E, Arín A, Valcayo A, Iñarrairaegui M, Nantes O, Prieto C (2004). "[Isotretinoin and ulcerous colitis]" (in Spanish; Castilian). An Sist Sanit Navar 27 (2): 241–3. PMID 15381956.
40. ^ Reniers DE, Howard JM (2001). "Isotretinoin-induced inflammatory bowel disease in an adolescent". Ann Pharmacother 35 (10): 1214–6. PMID 11675849. http://www.theannals.com/cgi/pmidlookup?view=long&pmid=11675849.
41. ^ "Jury Awards $10.5 Million Over Accutane". http://online.wsj.com/article/SB120890352155036159.html?mod=dist_smartbrief. Retrieved on 2008-06-21.
42. ^ Cobrin GM, Abreu MT (2005). "Defects in mucosal immunity leading to Crohn's disease". Immunol. Rev. 206: 277–95. doi:10.1111/j.0105-2896.2005.00293.x. PMID 16048555.
43. ^ a b Elson, C. (2007), "Monoclonal Anti–Interleukin 23 Reverses Active Colitis in a T Cell–Mediated Model in Mice", Gastroenterology 132: 2359, doi:10.1053/j.gastro.2007.03.104
44. ^ Prescott NJ, Fisher SA, Franke A, et al (2007). "A nonsynonymous SNP in ATG16L1 predisposes to ileal Crohn's disease and is independent of CARD15 and IBD5". Gastroenterology 132 (5): 1665–71. doi:10.1053/j.gastro.2007.03.034. PMID 17484864.
45. ^ {{cite journal| author= Marks DJ, Segal AW. |title=Innate immunity in inflammatory bowel disease: a disease hypothesis|journal= J Pathol. |volume =214 |issue= 4 |pages=260-6 |year=2008 |pmid= 18161747 |url=http://www.ncbi.nlm.nih.gov/pubmed/18161747?ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum
46. ^ {{cite journal| author= Marks DJ, Harbord MW, MacAllister R, Rahman FZ, Young J, Al-Lazikani B, Lees W, Novelli M, Bloom S, Segal AW |title=Defective acute inflammation in Crohn's disease: a clinical investigation |journal= Lancet |volume =367|issue= 9511 |pages=668-78 |year=2006 |pmid= 16503465 |http://www.ncbi.nlm.nih.gov/pubmed/16503465?ordinalpos=14&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum
47. ^ Dessein R, Chamaillard M, Danese S (2008). "Innate immunity in Crohn's disease: the reverse side of the medal". J Clin Gastroenterol 42 (Supplement 3): S144-7. PMID 18806708. http://www.ncbi.nlm.nih.gov/pubmed/18806708?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DiscoveryPanel.Pubmed_Discovery_RA&linkpos=4&log$=relatedreviews&logdbfrom=pubmed.
48. ^ "Crohns Research Overview Page". http://www.crohns.org/research/index.htm.
49. ^ Sartor, R. (2006). "Mechanisms of Disease: pathogenesis of Crohn's disease and ulcerative colitis". Nature Clinical Practice Gastroenterology & Hepatology (3): 390–407. doi:10.1038 (inactive 2008-06-25).
50. ^ Naser SA, Collins MT (2005). "Debate on the lack of evidence of Mycobacterium avium subsp. paratuberculosis in Crohn's disease". Inflamm. Bowel Dis. 11 (12): 1123. PMID 16306778.
51. ^ Giaffer MH, Clark A, Holdsworth CD (1992). "Antibodies to Saccharomyces cerevisiae in patients with Crohn's disease and their possible pathogenic importance". Gut 33 (8): 1071–5. doi:10.1136/gut.33.8.1071. PMID 1398231.
52. ^ Cite error: Invalid tag; no text was provided for refs named scah_out38
53. ^ "Paratuberculosis And Crohn's Disease: Got Milk?". http://www.mad-cow.org/00/paraTB.html.
54. ^ "Two-year outcomes analysis of Crohn's Disease treated with rifabutin and macrolide antibiotics.". http://alan.kennedy.name/crohns/research/chemo/htfull.htm.
55. ^ Baumgart, M., et al. (2007). "Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum (advance online publication)". The ISME Journal 1: 403. doi:10.1038/ismej.2007.52. http://search.nature.com/search/?sp-a=sp1001702d&sp-sfvl-field=subject. – Scholar search
56. ^ "Scientists find how bacteria in cows milk may cause Crohn's disease". University of Liverpool. http://liv.ac.uk/news/press_releases/2007/12/crohns-disease.htm.
57. ^ Cenac N, Andrews CN, Holzhausen M, et al (March 2007). "Role for protease activity in visceral pain in irritable bowel syndrome". J. Clin. Invest. 117 (3): 636–47. doi:10.1172/JCI29255. PMID 17304351.
58. ^ Cenac N, Coelho AM, Nguyen C, et al (November 2002). "Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2". Am. J. Pathol. 161 (5): 1903–15. PMID 12414536. http://ajp.amjpathol.org/cgi/pmidlookup?view=long&pmid=12414536.
59. ^ Boorom KF, Smith H, Nimri L, et al (October 2008). "Oh my aching gut: irritable bowel syndrome, Blastocystis, and asymptomatic infection". Parasit Vectors 1 (1): 40. doi:10.1186/1756-3305-1-40. PMID 18937874.
60. ^ Hugot, Jean-Pierre; Alberti, Corinne; Berrebi, Dominique; Bingen, Edouard; Cézard, Jean-Pierre (2003-12-13), "Crohn's disease: the cold chain hypothesis", The Lancet 362 (9400): 2012-2015, doi:10.1016/S0140-6736(03)15024-6, http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(03)15024-6
61. ^ "Fridges blamed for Crohn's disease rise", Medical News TODAY, 2003-12-12, http://www.medicalnewstoday.com/articles/4849.php
62. ^ Forbes, Alastair; Kalantzis, Tommy (2006), "Crohn's disease : the cold chain hypothesis", International journal of colorectal disease (Berlin: Springer) 21 (5): 399-401, ISSN 0179-1958, http://cat.inist.fr/?aModele=afficheN&cpsidt=17825639
63. ^ Crawford JM. "The Gastrointestinal tract, Chapter 17". In Cotran RS, Kumar V, Robbins SL. Robbins Pathologic Basis of Disease: 5th Edition. W.B. Saunders and Company, Philadelphia, 1994.
64. ^ HCP: Pill Cam, Capsule Endoscopy, Esophageal Endoscopy
65. ^ a b Hara, Amy K.; Jonathan A. Leighton, Russell I. Heigh, Virender K. Sharma, Alvin C. Silva, Giovanni De Petris, Joseph G. Hentz and David E. Fleischer (2006). "Crohn disease of the small bowel: preliminary comparison among CT enterography, capsule endoscopy, small-bowel follow-through, and ileoscopy". Radiology 238 (1): 128–34. doi:10.1148/radiol.2381050296. PMID 16373764.
66. ^ Triester, Stuart L.; Jonathan A. Leighton, Grigoris I. Leontiadis, Suryakanth R. Gurudu, David E. Fleische, Amy K. Hara, Russell I. Heigh, Arthur D. Shiff, and Virender K. Sharma (2006). "A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn's disease. This cannot and should not be used if a stricture or blockage is known, pictures should be taken prior to using the camera since it can get stuck and surgery in the only way to remove it". The American Journal of Gastroenterology 101 (5): 954–64. doi:10.1111/j.1572-0241.2006.00506.x. PMID 16696781.
67. ^ Dixon, P.M.; M.E. Roulston and D.J. Nolan (1993). "The small bowel enema: a ten year review". Clinical Radiology 47 (1): 46–8. doi:10.1016/S0009-9260(05)81213-9. PMID 8428417.
68. ^ Carucci, L. R.; M. S. Levine (2002). "Radiographic imaging of inflammatory bowel disease". Gastroenterology Clinics of North America 31 (1): 93–117. doi:10.1016/S0889-8553(01)00007-3. PMID 12122746.
69. ^ Rajesh, A.; D.D.T. Maglinte (2006). "Multislice CT enteroclysis: technique and clinical applications". Clinical Radiology 61 (1): 31–9. doi:10.1016/j.crad.2005.08.006. PMID 16356814.
70. ^ Zissin, Rivka; Marjorie Hertz, Alexandra Osadchy, Ben Novis and Gabriela Gayer (2005). "Computed Tomographic Findings of Abdominal Complications of Crohn’s Disease—Pictorial Essay" (PDF). Canadian Association of Radiologists Journal 56 (1): 25–35. PMID 15835588. http://www.carj.ca/issues/2005-Feb/25/pg25.pdf. Retrieved on 2006-07-02.
71. ^ MacKalski, B. A.; C. N. Bernstein (2005). "New diagnostic imaging tools for inflammatory bowel disease". Gut 55 (5): 733–41. doi:10.1136/gut.2005.076612. PMID 16609136.
72. ^ Goh, Jason; C. A. O'Morain (2003). "Review article: nutrition and adult inflammatory bowel disease". Alimentary Pharmacology & Therapeutics 17 (3): 307–20. doi:10.1046/j.1365-2036.2003.01482.x. PMID 12562443.
73. ^ Chamouard, Patrick; Zoe Richert, Nicolas Meyer, Gabriel Rahmi, René Baumann (2006). "Diagnostic Value of C-Reactive Protein for Predicting Activity Level of Crohn's Disease". Clinical Gastroenterology and Hepatology 4: 882. doi:10.1016/j.cgh.2006.02.003. PMID 16630759. Epub ahead of print
74. ^ Kaila, B.; K. Orr and C. N. Bernstein (2005). "The anti-Saccharomyces cerevisiae antibody assay in a province-wide practice: accurate in identifying cases of Crohn's disease and predicting inflammatory disease". The Canadian Journal of Gastroenterology 19 (12): 717–21. PMID 16341311. http://www.pulsus.com/Gastro/19_12/kail_ed.htm. Retrieved on 2006-07-02.
75. ^ Israeli, E.; I. Grotto, B. Gilburd, R. D. Balicer, E. Goldin, A. Wiik and Y. Shoenfeld (2005). "Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease". Gut 54 (9): 1232–6. doi:10.1136/gut.2004.060228. PMID 16099791.
76. ^ Faecal calprotectin: a bright future for assessing disease activity in Crohn's disease. Gaya et al. Q J Med 2002; 95: 557-558
77. ^ a b c d Kornbluth, Asher; David B. Sachar (2004). "Ulcerative Colitis Practice Guidelines in Adults" (PDF). American Journal of Gastroenterology 99 (7): 1371–85. doi:10.1111/j.1572-0241.2004.40036.x. PMID 15233681. http://www.acg.gi.org/physicians/guidelines/UlcerativeColitisUpdate.pdf. Retrieved on 2006-11-08.
78. ^ a b c d e Hanauer, Stephen B.; William Sandborn (2001-03-01). "Management of Crohn's Disease in Adults" (PDF). American Journal of Gastroenterology 96 (3): 635–43. doi:10.1111/j.1572-0241.2001.03671.x. PMID 11280528. http://www.acg.gi.org/physicians/guidelines/CrohnsDiseaseinAdults.pdf. Retrieved on 2006-11-08.
79. ^ Broomé, Ulrika; Annika Bergquist (2006). "Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer". Seminars in Liver Disease 26 (1): 31–41. doi:10.1055/s-2006-933561. PMID 16496231.
80. ^ "Clinical Research Alliance Update" (pdf). Crohn's and Colitis Foundation of America. 2007-05-01. http://www.ccfa.org/ccfaprof/research-grant-opps/documents/May-2007-Newsletter.pdf. Retrieved on 2008-02-14.
81. ^ a b Fries, WS (2007-05-16). "Crohn's Disease: 54 Tips to Help You Manage". WebMD. http://www.webmd.com/digestive-disorders/features/crohns-disease-54-tips-to-help-you-manage?ecd=wnl_gid_120607. Retrieved on 2008-02-14.
82. ^ Sandborn, W.J.; Colombel, J.F.; Enns, R.; Feagan, B.G.; Hanauer, S.B.; Lawrance, I.C.; Panaccione, R.; Sanders, M.; Schreiber, S.; Targan, S.; Others, (2005). "Natalizumab Induction and Maintenance Therapy for Crohn's Disease". New England Journal of Medicine 353 (18): 1912. doi:10.1056/NEJMoa043335. PMID 16267322.
83. ^ MacDonald, J.K.; McDonald, J.W.D. (2006). "Natalizumab for induction of remission in Crohn's disease (Cochrane Review)". The Cochrane Database of Systematic Reviews: 1465–858. doi:10.1002/14651858.CD006097. http://dellboy.update-software.com/abstracts/AB006097.htm. Retrieved on 2008-02-15.
84. ^ Longmore, Murray; Ian Wilkinson, Tom Turmezei, Chee Kay Cheung (2007). Oxford Handbook of Clinicial Medicine, 7th edition. Oxford University Press. pp. 266–7. ISBN 0-19-856837-1.
85. ^ Tresca, AJ (2007-01-12). "Resection Surgery for Crohn's Disease". About.com. http://ibdcrohns.about.com/od/surgeryprocedures/a/resectioncrohns.htm. Retrieved on 2008-02-14.
86. ^ Ozuner G, Fazio VW, Lavery IC, Milsom JW, Strong SA (1996). "Reoperative rates for Crohn's disease following strictureplasty. Long-term analysis". Dis. Colon Rectum 39 (11): 1199–203. doi:10.1007/BF02055108. PMID 8918424.
87. ^ Short Bowel Syndrome as defined by the National Institute of Diabetes and Digestive and Kidney Diseases
88. ^ Segal AW et al (2006). "Defective acute inflammation in Crohn's disease: a clinical investigation". Lancet 367 (9511): 668–78. doi:10.1016/S0140-6736(06)68265-2.
89. ^ Croese J, O'neil J, Masson J, et al (2006). "A proof of concept study establishing Necator americanus in Crohn's patients and reservoir donors". Gut 55 (1): 136–7. doi:10.1136/gut.2005.079129. PMID 16344586.
90. ^ Smith J, Stock H, Bingaman S et al (2007). "Low-Dose Naltrexone Therapy Improves Active Crohn’s Disease". American Journal of Gastroenterology 102 (4): 820–8. doi:10.1111/j.1572-0241.2007.01045.x. PMID 17222320.
91. ^ B. Omera, S. Krebsb, H. Omerc and T.O. Noord (2007). "Steroid-sparing effect of wormwood (Artemisia absinthium) in Crohn's disease: A double-blind placebo-controlled study". Phytomedicine 14 (2-3): 87–95. doi:10.1016/j.phymed.2007.01.001.
92. ^ "Bacterium 'to blame for Crohn's'". news.bbc.co.uk. http://news.bbc.co.uk/2/hi/health/7679347.stm. Retrieved on Nov. 23,2008.
93. ^ Canavan, C.; K. R. Abrams, J. Mayberry (2006). "Meta-analysis : colorectal and small bowel cancer risk in patients with Crohn's disease". Alimentary pharmacology & therapeutics 23 (8): 1097–104. doi:10.1111/j.1365-2036.2006.02854.x. ISSN 0269-2813. http://cat.inist.fr/?aModele=afficheN&cpsidt=17660183. Retrieved on 2007-05-23.
94. ^ a b Hiatt, Robert A.; Leon Kaufman (1988). "Epidemiology of inflammatory bowel disease in a defined northern California population". Western Journal of Medicine 149 (5): 541–6. PMID 3250100.
95. ^ Moum, B.; M. H. Vatn, A. Ekbom, E. Aadland, O. Fausa, I. Lygren, N. Stray, J. Sauar, T. Schulz (1996). "Incidence of Crohn's disease in four counties in southeastern Norway, 1990-93. A prospective population-based study. The Inflammatory Bowel South-Eastern Norway (IBSEN) Study Group of Gastroenterologists". Scandinavian Journal of Gastroenterology 31 (4): 355–61. doi:10.3109/00365529609006410. PMID 8726303.
96. ^ Shivananda, S.; J. Lennard-Jones, R. Logan, N. Fear, A. Price, L. Carpenter and M. van Blankenstein (1996). "Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD)". Gut 39 (5): 690–7. doi:10.1136/gut.39.5.690. PMID 9014768.
97. ^ "Crohn's disease manifests differently in boys and girls". CCFA.org. http://www.ccfa.org/reuters/ibdboysgirls.
98. ^ "Who is affected by Crohn's disease". WebMD.com. http://www.webmd.com/hw-popup/who-is-affected-by-crohns-disease.
99. ^ Satsangi J, Jewell DP, Bell JI (1997). "The genetics of inflammatory bowel disease". Gut 40 (5): 572–4. PMID 9203931. PMC: 1027155. http://gut.bmj.com/cgi/pmidlookup?view=long&pmid=9203931.
100. ^ Tysk C, Lindberg E, Järnerot G, Flodérus-Myrhed B (1988). "Ulcerative colitis and Crohn's disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking". Gut 29 (7): 990–6. PMID 3396969. PMC: 1433769. http://gut.bmj.com/cgi/pmidlookup?view=long&pmid=3396969.
101. ^ Kirsner JB (1988). "Historical aspects of inflammatory bowel disease". J. Clin. Gastroenterol. 10 (3): 286–97. PMID 2980764.
From Wikipedia, the free encyclopedia
Jump to: navigation, search
Crohn's disease
Classification and external resources
The three most common sites of intestinal involvement in Crohn's disease are
ileal, ileocolic and colonic.[1]
ICD-10
K50.
ICD-9
555
OMIM
266600
DiseasesDB
3178
MedlinePlus
000249
eMedicine
med/477 ped/507 radio/197
MeSH
D003424
Crohn's disease (also known as granulomatous colitis and regional enteritis) is an inflammatory disease which may affect any part of the gastrointestinal tract from mouth to anus, causing a wide variety of symptoms. It primarily causes abdominal pain, diarrhea (which may be bloody), vomiting, or weight loss,[1][2][3] but may also cause complications outside of the gastrointestinal tract such as skin rashes, arthritis and inflammation of the eye.[1]
Crohn's disease is an autoimmune disease, caused by the immune system attacking the gastrointestinal tract and producing inflammation in the gastrointestinal tract; it is classified as a type of inflammatory bowel disease. There appears to be a genetic link to Crohn's disease, with the highest risk occurring in individuals with siblings who have the disease. Males and females are equally affected. Smokers are three times more likely to develop Crohn's disease. [4] Crohn's disease affects between 400,000 and 600,000 people in North America.[5] Prevalence estimates for Northern Europe have ranged from 27–48 per 100,000.[6] Crohn's disease tends to present initially in the teens and twenties, with another peak incidence in the fifties to seventies, although the disease can occur at any age.[1][7]
There is no known drug or surgical cure for Crohn's disease;[8] treatment options are restricted to controlling symptoms, maintaining remission and preventing relapse.
The disease was independently described in 1904 by Polish surgeon Antoni Leśniowski and in 1932 by American gastroenterologist Burrill Bernard Crohn, for whom the disease was named. Crohn, along with two colleagues, described a series of patients with inflammation of the terminal ileum, the area most commonly affected by the illness.[9] For this reason, the disease has also been called regional ileitis[9] or regional enteritis.
Classification
Distribution of gastrointestinal Crohn's disease. Based on data from American Gastroenterological Association.
Crohn's disease is generally classified as an autoimmune disease. It invariably affects the gastrointestinal tract, and most gastroenterologists categorize the presenting disease by the affected areas. Ileocolic Crohn's disease, which affects both the ileum (the last part of the small intestine that connects to the large intestine) and the large intestine, accounts for fifty percent of cases. Crohn's ileitis, affecting the ileum only, accounts for thirty percent of cases, and Crohn's colitis, affecting the large intestine, accounts for the remaining twenty percent of cases and may be particularly difficult to distinguish from ulcerative colitis. The disease can attack any part of the digestive tract, from mouth to anus. However, individuals affected by the disease rarely fall outside these three classifications, being affected in other parts of the gastrointestinal tract such as the stomach and esophagus.[1] Crohn's disease may also be categorized by the behavior of disease as it progresses. This was formalized in the Vienna classification of Crohn's disease.[10] There are three categories of disease presentation in Crohn's disease: stricturing, penetrating, and inflammatory. Stricturing disease causes narrowing of the bowel which may lead to bowel obstruction or changes in the caliber of the feces. Penetrating disease creates abnormal passageways (fistulae) between the bowel and other structures such as the skin. Inflammatory disease (or non-stricturing, non-penetrating disease) causes inflammation without causing strictures or fistulae.[10][11]
Symptoms
Endoscopy image of colon showing serpiginous ulcer, a classic finding in Crohn's disease
Many people with Crohn's disease have symptoms for years prior to the diagnosis.[12] The usual onset is between 15 and 30 years of age but can occur at any age.[13] Because of the 'patchy' nature of the gastrointestinal disease and the depth of tissue involvement, initial symptoms can be more vague than with ulcerative colitis. People with Crohn's disease will go through periods of flare-ups and remission.
Gastrointestinal symptoms
Abdominal pain may be the initial symptom of Crohn's disease. It is often accompanied by diarrhea, which may or may not be bloody, though diarrhea is not uncommon especially in those who have had surgery. People who have had surgery or multiple surgeries often end up with short bowel syndrome of the gastrointestinal tract. The nature of the diarrhea in Crohn's disease depends on the part of the small intestine or colon that is involved. Ileitis typically results in large-volume watery feces. Colitis may result in a smaller volume of feces of higher frequency. Fecal consistency may range from solid to watery. In severe cases, an individual may have more than 20 bowel movements per day and may need to awaken at night to defecate.[1][7][14][15] Visible bleeding in the feces is less common in Crohn's disease than in ulcerative colitis, but may be seen in the setting of Crohn's colitis.[1] Bloody bowel movements are typically intermittent, and may be bright or dark red in colour. In the setting of severe Crohn's colitis, bleeding may be copious.[7] Flatulence and bloating may also add to the intestinal discomfort.[7]
Symptoms caused by intestinal stenosis are also common in Crohn's disease. Abdominal pain is often most severe in areas of the bowel with stenoses. In the setting of severe stenosis, vomiting and nausea may indicate the beginnings of small bowel obstruction.[7] Crohn's disease may also be associated with primary sclerosing cholangitis, a type of inflammation of the bile ducts.[citation needed]
Perianal discomfort may also be prominent in Crohn's disease. Itchiness or pain around the anus may be suggestive of inflammation, fistulization or abscess around the anal area[1] or anal fissure. Perianal skin tags are also common in Crohn's disease.[16] Fecal incontinence may accompany peri-anal Crohn's disease. At the opposite end of the gastrointestinal tract, the mouth may be affected by non-healing sores (aphthous ulcers). Rarely, the esophagus, and stomach may be involved in Crohn's disease. These can cause symptoms including difficulty swallowing (odynophagia), upper abdominal pain, and vomiting.[17]
Systemic symptoms
Crohn's disease, like many other chronic, inflammatory diseases, can cause a variety of systemic symptoms.[1] Among children, growth failure is common. Many children are first diagnosed with Crohn's disease based on inability to maintain growth.[18] As Crohn's disease may manifest at the time of the growth spurt in puberty, up to 30% of children with Crohn's disease may have retardation of growth.[19] Fever may also be present, though fevers greater than 38.5 ˚C (101.3 ˚F) are uncommon unless there is a complication such as an abscess[1] Among older individuals, Crohn's disease may manifest as weight loss. This is usually related to decreased food intake, since individuals with intestinal symptoms from Crohn's disease often feel better when they do not eat and might lose their appetite.[18] People with extensive small intestine disease may also have malabsorption of carbohydrates or lipids, which can further exacerbate weight loss.[20]
Extraintestinal symptoms
Erythema nodosum
In addition to systemic and gastrointestinal involvement, Crohn's disease can affect many other organ systems.[21] Inflammation of the interior portion of the eye, known as uveitis, can cause eye pain, especially when exposed to light (photophobia). Inflammation may also involve the white part of the eye (sclera), a condition called episcleritis. Both episcleritis and uveitis can lead to loss of vision if untreated.
Crohn's disease is associated with a type of rheumatologic disease known as seronegative spondyloarthropathy. This group of diseases is characterized by inflammation of one or more joints (arthritis) or muscle insertions (enthesitis). The arthritis can affect larger joints such as the knee or shoulder or may exclusively involve the small joints of the hand and feet. The arthritis may also involve the spine, leading to ankylosing spondylitis if the entire spine is involved or simply sacroiliitis if only the lower spine is involved. The symptoms of arthritis include painful, warm, swollen, stiff joints and loss of joint mobility or function.[citation needed]
Crohn's disease may also involve the skin, blood, and endocrine system. One type of skin manifestation, erythema nodosum, presents as red nodules usually appearing on the shins. Erythema nodosum is due to inflammation of the underlying subcutaneous tissue and is characterized by septal panniculitis. Another skin lesion, pyoderma gangrenosum, is typically a painful ulcerating nodule. Crohn's disease also increases the risk of blood clots; painful swelling of the lower legs can be a sign of deep venous thrombosis, while difficulty breathing may be a result of pulmonary embolism. Autoimmune hemolytic anemia, a condition in which the immune system attacks the red blood cells, is also more common in Crohn's disease and may cause fatigue, pallor, and other symptoms common in anemia. Clubbing, a deformity of the ends of the fingers, may also be a result of Crohn's disease. Finally, Crohn's disease may cause osteoporosis, or thinning of the bones. Individuals with osteoporosis are at increased risk of bone fractures.[6]
Crohn's disease can also cause neurological complications (reportedly in up to 15% of patients).[22] The most common of these are seizures, stroke, myopathy, peripheral neuropathy, headache and depression.[22]
Crohn's patients often also have issues with Small bowel bacterial overgrowth syndrome, which has similar symptoms.[23]
Complications
Endoscopic image of colon cancer identified in the sigmoid colon on screening colonoscopy for Crohn's disease.
Crohn's disease can lead to several mechanical complications within the intestines, including obstruction, fistulae, and abscesses. Obstruction typically occurs from strictures or adhesions which narrow the lumen, blocking the passage of the intestinal contents. Fistulae can develop between two loops of bowel, between the bowel and bladder, between the bowel and vagina, and between the bowel and skin. Abscesses are walled off collections of infection, which can occur in the abdomen or in the perianal area in Crohn's disease sufferers.
Crohn's disease also increases the risk of cancer in the area of inflammation. For example, individuals with Crohn's disease involving the small bowel are at higher risk for small intestinal cancer. Similarly, people with Crohn's colitis have a relative risk of 5.6 for developing colon cancer.[24] Screening for colon cancer with colonoscopy is recommended for anyone who has had Crohn's colitis for eight years, or more.[25] Some study suggest that there is a role for chimioprotection in the prevention of colorectal cancer in Crohn's involving the colon; two agents have been suggested, folate and mesalamine preparations.[26]
Individuals with Crohn's disease are at risk of malnutrition for many reasons, including decreased food intake and malabsorption. The risk increases following resection of the small bowel. Such individuals may require oral supplements to increase their caloric intake, or in severe cases, total parenteral nutrition (TPN). Most people with moderate or severe Crohn's disease are referred to a dietitian for assistance in nutrition.[27]
Crohn's disease can cause significant complications including bowel obstruction, abscesses, free perforation and hemorrhage.[28]
Crohn's disease can be problematic during pregnancy, and some medications can cause adverse outcomes for the fetus or mother. Consultation with an obstetrician and gastroenterologist about Crohn's disease and all medications allows preventative measures to be taken. In some cases, remission can occur during pregnancy. Certain medications can also impact sperm count or may otherwise adversely affect a man's ability to conceive.[29]
Cause
The exact cause of Crohn's disease is unknown. Environmental and genetic factors have been invoked in the pathogenesis of the disease.
Genetics
Schematic of NOD2 CARD15 gene, which is associated with certain disease patterns in Crohn's disease.
Research has indicated that Crohn's disease has a strong genetic link.[30] The disease runs in families and those with a sibling with the disease are 30 times more likely to develop it than the normal population. Ethnic background is also a risk factor.
Mutations in the CARD15 gene (also known as the NOD2 gene) are associated with Crohn's disease[31] and with susceptibility to certain phenotypes of disease location and activity.[32] In earlier studies, only two genes were linked to Crohn's, but scientists now believe there are over eight genes that show genetics play a role in the disease, either directly through causation or indirectly as with a mediator variable. Anomalies in the XBP1 gene have recently been identified as a factor, pointing towards a role for the unfolded protein response pathway of the endoplasmatic reticulum in inflammatory bowel diseases.[33][34]
Environmental factors
A handful of cases of Crohn's disease cases were reported at the turn of the 20th century, but since then, the disease has continued to increase in prevalence dramatically. Some argue that this increase has been the result of a genetic shift in the population caused by conditions favoring individuals carrying the genes linked with the disease. These conditions could be a lower infant mortality rate or better health care in the nations that have the highest incidence of disease (industrialized nations). Another explanation is that modern industrial practices have given rise to increased disease prevalence via infectious diseases. A common recurrent theory is that a specific species of Mycobacterium, Mycobacterium avium subspecies paratuberculosis, is responsible for both Johne's disease and Crohn's disease via modern industrial farming practices.[citation needed]
Smoking has been shown to increase the risk of the return of active disease, or "flares".[35] The introduction of hormonal contraception in the United States in the 1960s is linked with a dramatic increase in the incidence rate of Crohn's disease. Although a causal linkage has not been effectively shown, there remain fears that these drugs work on the digestive system in similar ways to smoking.[36]
Some scientific studies and court rulings have posited that Accutane is a probable cause of IBD, a group of diseases including Crohn's Disease and Ulcerative colitis, in some individuals. Three cases in the United States have gone to trial thus far, with all three resulting in multi-million dollar judgments against the makers of isotretinoin; there are an additional 425 cases pending. [37] [38] [39] [40] [41]
Immune system
Abnormalities in the immune system have often been invoked as being causes of Crohn's disease. Crohn's disease is thought to be an autoimmune disease, with inflammation stimulated by an over-active Th1 cytokine response.[42] However, more recent evidence has shown that Th17 is of greater importance in the disease.[43] The most recent gene to be implicated in Crohn's disease is ATG16L1, which may reduce the effectiveness of autophagy, and hinder the body's ability to attack invasive bacteria.[44]
Contrary to the prevailing view that Crohn's disease is a primary T cell autoimmune disorder, there is an increasing body of evidence in favour of the hypothesis that Crohn's disease results from an impaired innate immunity.[45] The immunodeficiency, which has been shown to be due to (at least in part) impaired cytokine secretion by macrophages,[46] is thought to lead to a sustained microbial-induced inflammatory response, particularly in the colon where the bacterial load is especially high. [47]
Microbes
A variety of pathogenic bacteria were initially suspected of being causative agents of Crohn's disease and many groups still suspect Mycobacterium avium subspecies paratuberculosis.[48] However, the consensus of many health care professionals for several years has been that a variety of microorganisms are simply taking advantage of their host's weakened mucosal layer and inability to clear bacteria from the intestinal walls, both symptoms of the disease.[49] Some studies have linked Mycobacterium avium subsp. paratuberculosis to Crohn's disease, in part because it causes a very similar disease, Johne's disease, in cattle.[50] The mannose bearing antigens, mannins, from yeast may also elicit pathogenic anti saccharomyces cerevisiae antibodies.[51] Studies have linked specific strains of enteroadherent E. coli to the disease. Still this relationship and evidence of contributions by other species remains controversial.[52][53][54][55] And more recently a relationship between MAP and E. coli has been uncovered. Scientists at the university of Liverpool have discovered that "Mycobacteria release a complex molecule containing a sugar, called mannose. This molecule prevents a type of white blood cells, called macrophages, from killing internalized E.Coli.”[56]
Some studies have suggested that Crohn's disease, ulcerative colitis and irritable bowel syndrome have the same underlying cause. Biopsy samples taken from the colons of all three patient groups were found to produce elevated levels of a serine protease. [57] Experimental introduction of the serine protease into mice has been found to produce widespread pain associated with irritable bowel syndrome as well as colitis, which is associated with all three diseases.[58] The authors of that study were unable to identify the source of the protease, but a separate review noted that regional and temporal variations in those illnesses follow those associated with infection with a poorly understood protozoan, Blastocystis. [59]
A study in 2003 put forth the "cold-chain" hypothesis, that psychrotrophic bacteria such as Yersinia spp and Listeria spp contribute to the disease. A statistical correlation was found between the advent of the use of refrigeration in the United States and various parts of Europe and the rise of the disease. [60] [61] Later studies have provided support for this hypothesis[62].
Pathophysiology
Section of colectomy showing transmural inflammation
At the time of colonoscopy, biopsies of the colon are often taken in order to confirm the diagnosis. There are certain characteristic features of the pathology seen that point toward Crohn's disease. Crohn's disease shows a transmural pattern of inflammation, meaning that the inflammation may span the entire depth of the intestinal wall.[1] Grossly, ulceration is an outcome seen in highly active disease. There is usually an abrupt transition between unaffected tissue and the ulcer. Under a microscope, biopsies of the affected colon may show mucosal inflammation. This inflammation is characterized by focal infiltration of neutrophils, a type of inflammatory cell, into the epithelium. This typically occurs in the area overlying lymphoid aggregates. These neutrophils, along with mononuclear cells, may infiltrate into the crypts leading to inflammation (crypititis) or abscess (crypt abscess). Granulomas, aggregates of macrophage derivatives known as giant cells, are found in 50% of cases and are most specific for Crohn's disease. The granulomas of Crohn's disease do not show "caseation", a cheese-like appearance on microscopic examination that is characteristic of granulomas associated with infections such as tuberculosis. Biopsies may also show chronic mucosal damage as evidenced by blunting of the intestinal villi, atypical branching of the crypts, and change in the tissue type (metaplasia). One example of such metaplasia, Paneth cell metaplasia, involves development of Paneth cells (typically found in the small intestine) in other parts of the gastrointestinal system.[63]
According to the volume 1 of the 10th Edition of Brunner and Suddarth’s Textbook of Medical-Surgical Nursing Regional enteritis is a subacute and chronic inflammation that extends through the layers (ie, transmural lesion) of the bowel wall from the intestinal mucosa. It is characterized by periods of remissions and exacerbations. The disease process begins with edema and thickening of the mucosa. Ulcers begin to appear on the inflamed mucosa. These lesions are not in continuous contact with one another and are separated by normal tissue. Fistulas, fissures, and abscesses form as the inflammation extends into the peritoneum. Granulomas occur in one half of patients. In advanced cases, the intestinal mucosa has a cobblestone appearance. As the disease advances, the bowel wall thickens and becomes fibrotic, and the intestinal lumen narrows. Diseased bowel loops sometimes adhere to other loops surrounding them.
Diagnosis
Endoscopic image of Crohn's colitis showing deep ulceration
CT scan showing Crohn's disease in the fundus of the stomach
Crohn's disease can mimic ulcerative colitis on endoscopy. This endoscopic image is of Crohn's colitis showing diffuse loss of mucosal architecture, friability of mucosa in sigmoid colon and exudate on wall, all of which can be found with ulcerative colitis.
The diagnosis of Crohn's disease can sometimes be challenging,[12] and a number of tests are often required to assist the physician in making the diagnosis.[7] Even with a full battery of tests it may not be possible to diagnose Crohn's with complete certainty; a colonoscopy is approximately 70% effective in diagnosing the disease with further being less effective. Disease in the small bowel is particularly difficult to diagnose as a traditional colonoscopy only allows access to the colon and lower portions of the small intestines; introduction of the Capsule endoscopy[64] aids in endoscopic diagnosis.
Endoscopy
A colonoscopy is the best test for making the diagnosis of Crohn's disease as it allows direct visualization of the colon and the terminal ileum, identifying the pattern of disease involvement. Occasionally, the colonoscope can travel past the terminal ileum but it varies from patient to patient. During the procedure, the gastroenterologist can also perform a biopsy, taking small samples of tissue for laboratory analysis which may help confirm a diagnosis. As 30% of Crohn's disease involves only the ileum,[1] cannulation of the terminal ileum is required in making the diagnosis. Finding a patchy distribution of disease, with involvement of the colon or ileum but not the rectum, is suggestive of Crohn's disease, as are other endoscopic stigmata.[65]The utility of capsule endoscopy for this, however, is still uncertain.[66]
Radiologic tests
A small bowel follow-through may suggest the diagnosis of Crohn's disease and is useful when the disease involves only the small intestine. Because colonoscopy and gastroscopy allow direct visualization of only the terminal ileum and beginning of the duodenum, they cannot be used to evaluate the remainder of the small intestine. As a result, a barium follow-through x-ray, wherein barium sulfate suspension is ingested and fluoroscopic images of the bowel are taken over time, is useful for looking for inflammation and narrowing of the small bowel.[65][67] Barium enemas, in which barium is inserted into the rectum and fluoroscopy used to image the bowel, are rarely used in the work-up of Crohn's disease due to the advent of colonoscopy. They remain useful for identifying anatomical abnormalities when strictures of the colon are too small for a colonoscope to pass through, or in the detection of colonic fistulae.[68]
CT and MRI scans are useful for evaluating the small bowel with enteroclysis protocols.[69]They are additionally useful for looking for intra-abdominal complications of Crohn's disease such as abscesses, small bowel obstruction, or fistulae.[70] Magnetic resonance imaging (MRI) are another option for imaging the small bowel as well as looking for complications, though it is more expensive and less readily available[71]
Blood tests
A complete blood count may reveal anemia, which may be caused either by blood loss or vitamin B12 deficiency. The latter may be seen with ileitis because vitamin B12 is absorbed in the ileum.[72] Erythrocyte sedimentation rate, or ESR, and C-reactive protein measurements can also be useful to gauge the degree of inflammation.[73] It is also true in patient with ilectomy done in response to the complication. Another cause of anaemia is anaemia of chronic disease, characterized by its microcytic and hypochromic anaemia. There can be various reasons for anaemia, including medication used in treatment of inflammatory bowel disease like azathioprine which can lead to cytopenia and sulfasalazine which can also result in folate malabsorption, etc. Testing for anti-Saccharomyces cerevisiae antibodies (ASCA) and anti-neutrophil cytoplasmic antibodies (ANCA) has been evaluated to identify inflammatory diseases of the intestine[74] and to differentiate Crohn's disease from ulcerative colitis.[75]
Feces
Calprotectin is a protein found in neutrophil cytosol. Neutrophils are integral to the inflammatory process caused by Crohn's disease. Faecal Calprotectin is a protein measured in a patient's feces. The fecal concentration correlates well with inflammation and disease activity. Normal fecal calprotectin level in a patient with active gastrointestinal symptoms excludes inflammatory bowel disease as a likely diagnosis and in many cases negates the need for colonoscopy or radio labelled white cell scanning[76].
Comparison with ulcerative colitis
The most common disease that mimics the symptoms of Crohn's disease is ulcerative colitis, as both are inflammatory bowel diseases that can affect the colon with similar symptoms. It is important to differentiate these diseases, since the course of the diseases and treatments may be different. In some cases, however, it may not be possible to tell the difference, in which case the disease is classified as indeterminate colitis.[14][1][7]
Comparisons of various factors in Crohn's disease and ulcerative colitis
Crohn's disease Ulcerative colitis
Terminal ileum involvement Commonly Seldom
Colon involvement Usually Always
Rectum involvement Seldom Usually[77]
Involvement around the anus
Common[78]
Seldom
Bile duct involvement No increase in rate of primary sclerosing cholangitis
Higher rate[79]
Distribution of Disease Patchy areas of inflammation (Skip lesions) Continuous area of inflammation[77]
Endoscopy Deep geographic and serpiginous (snake-like) ulcers
Continuous ulcer
Depth of inflammation May be transmural, deep into tissues[78][1]
Shallow, mucosal
Fistulae
Common[78]
Seldom
Stenosis
Common Seldom
Autoimmune disease
Widely regarded as an autoimmune disease No consensus
Cytokine response
Associated with Th17 [43]
Vaguely associated with Th2
Granulomas on biopsy Can have granulomas[78]
Granulomas not seen[77]
Surgical cure Often returns following removal of affected part Usually cured by removal of colon
Smoking
Higher risk for smokers Lower risk for smokers[77]
Treatment
Main articles: Treatment of Crohn's disease and Biological therapy for inflammatory bowel disease
Currently there is no cure for Crohn's disease and remission may not be possible or prolonged if achieved, although in recent studies low dose naltroxen has shown improvement in Crohn's disease.;[80] in cases where remission is possible, relapse can be prevented and symptoms controlled with medication, lifestyle changes and in some cases, surgery. Adequately controlled, Crohn's disease may not significantly restrict daily living.[81] Treatment for Crohn's disease is only when symptoms are active and involve first treating the acute problem, then maintaining remission.
Medication
Acute treatment uses medications to treat any infection (normally antibiotics) and to reduce inflammation (normally aminosalicylate anti-inflammatory drugs and corticosteroids). When symptoms are in remission, treatment enters maintenance with a goal of avoiding the recurrence of symptoms. Prolonged use of corticosteroids has significant side-effects; as a result they are generally not used for long-term treatment. Alternatives include aminosalicylates alone, though only a minority are able to maintain the treatment, and many require immunosuppressive drugs.[78]
Medications used to treat the symptoms of Crohn's disease include 5-aminosalicylic acid (5-ASA) formulations, prednisone, immunomodulators such as azathioprine, mercaptopurine, methotrexate, infliximab, adalimumab[14] and natalizumab.[82][83] Hydrocortisone should be used in severe attacks of Crohn's disease.[84]
Lifestyle changes
Certain lifestyle changes can reduce symptoms, including dietary adjustments, proper hydration and smoking cessation. Eating small meals frequently instead of big meals may help with a low appetite. To manage symptoms have a balanced diet with proper portion control. Fatigue can be helped with regular excercise, a healthy diet and enough sleep. A food diary may help with identifying foods that trigger symptoms. Some patients should follow a low fiber diet to control symptoms especially if fiberous foods cause symptoms.[81]
Surgery
Crohn's cannot be cured by surgery, though it is used when partial or a full blockage of the intestine occurs. Surgery may also be required for complications such as obstructions, fistulas and/or abscesses, or if the disease does not respond to drugs. After the first surgery, Crohn's usually shows up at the site of the resection though it can appear in other locations. After a resection, scar tissue builds up which can cause strictures. A stricture is when the intestines becomes too small to allow excrement to pass through easily which can lead to a blockage. After the first resection, another resection may be necessary within five years.[85] For patients with an obstruction due to a stricture, two options for treatment are strictureplasty and resection of that portion of bowel. There is no statistical significance between strictureplasty alone versus strictureplasty and resection in cases of duodenal involvement. In these cases, re-operation rates were 31% and 27%, respectively, indicating that strictureplasty is a safe and effective treatment for selected patients with duodenal involvement.[86]
Short bowel syndrome (SBS, also short gut syndrome or simply short gut) can be caused by the surgical removal of the small intestines. It usually develops if a person has had half or more of their small intestines removed.[87] Diarrhea is the main symptom of short bowel syndrome though other symptoms may include cramping, bloating and heartburn. Short bowel syndrome is treated with changes in diet, intravenous feeding, vitamin and mineral supplements and treatment with medications. Another complication following surgery for Crohn's disease where the terminal ileum has been removed is the development of excessive watery diarrhoea. This is due to an inability to reabsorb bile acids after resection of the terminal ileum.[citation needed]
Prospective treatments
Researchers at University College London have questioned the wisdom of suppressing the immune system in Crohn's, as the problem may be an under-active rather than an over-active immune system: their study found that Crohn's patients showed an abnormally low response to an introduced infection, marked by a poor flow of blood to the wound, and the response improved when the patients were given sildenafil.[88]
Recent studies using helminthic therapy or hookworms to treat Crohn's Disease and other (non-viral) auto-immune diseases seem to yield promising results.[89]
A single, small, uncontrolled trial of patients with mild Crohn's on stable medications suggested improvement with low dose naltrexone therapy. Further studies are needed to confirm the use of this. [90]
A recent small double-blind, placebo-controlled study[91] conducted in Germany found grand wormwood to have a steroid-sparing effect with the result that 18/20 wormwood receiving patients were able to taper steroid medication while maintaining a steady improvement in CD symptoms.
Recent research in France has suggested that a shortage of the bacterium Faecalibacterium prausnitzii may cause Crohn's disease by overstimulating the immune system. The researchers said that if ongoing animal trials prove successful, human patients could benefit from a probiotic treatment with F. prausnitzii. However, it was stated "It is too early to tell whether this will lead directly to a new treatment as other probiotics have tended to produce good results in animal studies only to prove disappointing in clinical trial in Crohn's disease." [92]
Prognosis
Crohn's disease is a chronic condition for which there is currently no cure. It is characterised by periods of improvement followed by episodes when symptoms flare up. With treatment, most people achieve a healthy height and weight, and the mortality rate for the disease is low. Crohn's disease is associated with an increased risk of small bowel and colorectal carcinoma.[93]
Epidemiology
The incidence of Crohn's disease has been ascertained from population studies in Norway and the United States and is similar at 6 to 7.1:100,000.[94][95] Crohn's disease is more common in northern countries, and shows a higher preponderance in northern areas of the same country.[96] The incidence of Crohn's disease is thought to be similar in Europe but lower in Asia and Africa.[94] It also has a higher incidence in Ashkenazi Jews.[14]
Crohn's disease has a bimodal distribution in incidence as a function of age: the disease tends to strike people in their teens and 20s, and people in their 50s through to their 70s, and ages in between due to not being diagnosed with Crohn's and being diagnosed instead with irritable bowel syndrome (IBS).[1][7] It is rarely diagnosed in early childhood. It usually strikes females who are pediatric patients more severely than males.[97] However, only slightly more women than men have Crohn's disease.[98] Parents, siblings or children of people with Crohn's disease are 3 to 20 times more likely to develop the disease.[99] Twin studies show a concordance of greater than 55% for Crohn's disease.[100]
History
Inflammatory bowel diseases were described by Giovanni Battista Morgagni (1682-1771), by Polish surgeon Antoni Leśniowski in 1904 (leading to the use of the eponym "Leśniowski-Crohn disease" in Poland) and by Scottish physician T. Kennedy Dalziel in 1913.[101]
Burrill Bernard Crohn, an American gastroenterologist at New York City's Mount Sinai Hospital, described fourteen cases in 1932, and submitted them to the American Medical Association under the rubric of "Terminal ileitis: A new clinical entity". Later that year, he, along with colleagues Leon Ginzburg and Gordon Oppenheimer published the case series as "Regional ileitis: a pathologic and clinical entity".[9]
References
1. ^ a b c d e f g h i j k l m n o Baumgart DC, Sandborn WJ (2007). "Inflammatory bowel disease: clinical aspects and established and evolving therapies.". The Lancet 369 (9573): 1641–57. doi:10.1016/S0140-6736(07)60751-X. PMID 17499606.
2. ^ Mayo Clinic: Crohn's Disease
3. ^ National Digestive Diseases Information Clearinghouse
4. ^ http://www.ncbi.nlm.nih.gov/pubmed/15157822
5. ^ Loftus, E.V.; P. Schoenfeld, W. J. Sandborn (2002). "The epidemiology and natural history of Crohn's disease in population-based patient cohorts from North America: a systematic review". Alimentary Pharmacology & Therapeutics 16 (1): 51–60. doi:10.1046/j.1365-2036.2002.01140.x. PMID 11856078.
6. ^ a b Bernstein, Charles N. (2006). The Epidemiology of Inflammatory Bowel Disease in Canada: A Population-Based Study. 101. pp. 1559–68. doi:10.1111/j.1572-0241.2006.00603.x. PMID 16863561.
7. ^ a b c d e f g h Gopal, Latha; Senthil Nachimuthu (2006-05-23). "Crohn Disease". eMedicine. http://www.emedicine.com/MED/topic477.htm. Retrieved on 2006-07-02.
8. ^ Al-Ataie, M Bashar; Vishwanath N Shenoy (2005-10-04). "Ulcerative colitis". eMedicine. http://www.emedicine.com/med/topic2336.htm. Retrieved on 2006-07-02.
9. ^ a b c Crohn BB, Ginzburg L, Oppenheimer GD (2000). "Regional ileitis: a pathologic and clinical entity. 1932". Mt. Sinai J. Med. 67 (3): 263–8. PMID 10828911.
10. ^ a b Gasche C, Scholmerich J, Brynskov J, D'Haens G, Hanauer S, Irvine E, Jewell D, Rachmilewitz D, Sachar D, Sandborn W, Sutherland L (2000). "A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998". Inflamm Bowel Dis 6 (1): 8–15. PMID 10701144.
11. ^ Dubinsky MC, Fleshner PP. (2003). "Treatment of Crohn's Disease of Inflammatory, Stenotic, and Fistulizing Phenotypes". Curr Treat Options Gastroenterol 6 (3): 183–200. doi:10.1007/s11938-003-0001-1. PMID 12744819.
12. ^ a b Pimentel, Mark; Michael Chang, Evelyn J. Chow, Siamak Tabibzadeh, Viorelia Kirit-Kiriak, Stephan R. Targan, Henry C. Lin (2000). "Identification of a prodromal period in Crohn's disease but not ulcerative colitis". American Journal of Gastroenterology 95 (12): 3458–62. doi:10.1111/j.1572-0241.2000.03361.x. PMID 11151877.
13. ^ Crohn's Disease Overview
14. ^ a b c d Podolsky, Daniel K. (2002). "Inflammatory bowel disease". New England Journal of Medicine 346 (6): 417–29. doi:10.1056/NEJMra020831. PMID 12167685. http://content.nejm.org/cgi/content/extract/347/6/417. Retrieved on 2006-07-02.
15. ^ Mueller, M. H.; M. E. Kreis, M. L. Gross, H. D. Becker, T. T. Zittel & E. C. Jehle (2002). "Anorectal functional disorders in the absence of anorectal inflammation in patients with Crohn's disease". British Journal of Surgery 89 (8): 1027–31. doi:10.1046/j.1365-2168.2002.02173.x. PMID 12153630.
16. ^ Taylor B, Williams G, Hughes L, Rhodes J (1989). "The histology of anal skin tags in Crohn's disease: an aid to confirmation of the diagnosis". Int J Colorectal Dis 4 (3): 197–9. doi:10.1007/BF01649703. PMID 2769004.
17. ^ Fix, Oren K.; Jorge A. Soto, Charles W. Andrews and Francis A. Farraye (2004). "Gastroduodenal Crohn's disease". Gastrointestinel Endoscopy 60 (6): 985. doi:10.1016/S0016-5107(04)02200-X. PMID 15605018.
18. ^ a b Beattie, R.M.; N. M. Croft, J. M. Fell, N. A. Afzal and R. B. Heuschkel (2006). "Inflammatory bowel disease". Archives of Disease in Childhood 91 (5): 426–32. doi:10.1136/adc.2005.080481. PMID 16632672.
19. ^ Büller, H.A. (1997). "Problems in diagnosis of IBD in children". The Netherlands Journal of Medicine 50 (2): S8–S11. doi:10.1016/S0300-2977(96)00064-2. PMID 9050326.
20. ^ O'Keefe, S. J. (1996). "Nutrition and gastrointestinal disease". Scandinavian Journal of Gastroenterology Supplement 31 (220): 52–9. doi:10.3109/00365529609094750. PMID 8898436.
21. ^ Danese, Silvio; Stefano Semeraro, Alfredo Papa, Italia Roberto, Franco Scaldaferri, Giuseppe Fedeli, Giovanni Gasbarrini, Antonio Gasbarrini (2005). "Extraintestinal manifestations in inflammatory bowel disease". World Journal of Gastroenterology 11 (46): 7227–36. PMID 16437620. http://www.wjgnet.com/1007-9327/11/7227.asp. Retrieved on 2006-07-02.
22. ^ a b Crohn's disease. professionals.epilepsy.com. Retrieved on July 13, 2007.
23. ^ MedlinePlus Encyclopedia Small bowel bacterial overgrowth
24. ^ Ekbom A, Helmick C, Zack M, Adami H (1990). "Increased risk of large-bowel cancer in Crohn's disease with colonic involvement". Lancet 336 (8711): 357–9. doi:10.1016/0140-6736(90)91889-I. PMID 1975343.
25. ^ Collins P, Mpofu C, Watson A, Rhodes J (2006). "Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease". Cochrane Database Syst Rev: CD000279. doi:10.1002/14651858.CD000279.pub3. PMID 16625534.
26. ^ Lynne V McFarland (2008). "Colorectal cancer and dysplasia in inflammatory bowel disease". World Journal of Gastroenterology: 2665.
27. ^ Evans J, Steinhart A, Cohen Z, McLeod R (2003). "Home total parenteral nutrition: an alternative to early surgery for complicated inflammatory bowel disease". J Gastrointest Surg 7 (4): 562–6. doi:10.1016/S1091-255X(02)00132-4. PMID 12763417.
28. ^ "Complications of Crohn's Disease". https://www.livingwithcrohnsdisease.com/livingwithcrohnsdisease/crohns_disease/complications_of_crohns.html. Retrieved on 2008-01-16.
29. ^ Kaplan, C (2005-10-21). "IBD and Pregnancy: What You Need to Know". Crohn's and Colitis Foundation of America. http://www.ccfa.org/about/news/pregnancy. Retrieved on 2008-02-14.
30. ^ "Crohn's disease has strong genetic link: study". Crohn's and Colitis Foundation of America. 2007-04-16. http://www.ccfa.org/reuters/geneticlink. Retrieved on 2008-04-20.
31. ^ Ogura Y, Bonen DK, Inohara N, et al (2001). "A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease". Nature 411 (6837): 603–6. doi:10.1038/35079114. PMID 11385577.
32. ^ Cuthbert A, Fisher S, Mirza M, et al. (2002). "The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease". Gastroenterology 122 (4): 867–74. doi:10.1053/gast.2002.32415. PMID 11910337.
33. ^ Kaser et. al. (5 September 2008). "XBP1 Links ER Stress to Intestinal Inflammation and Confers Genetic Risk for Human Inflammatory Bowel Disease". Cell (Cell Press) 134: 743–756. http://www.cell.com/content/article/abstract?uid=PIIS0092867408009410.
34. ^ Clevers H (2009). "Inflammatory Bowel Disease, Stress, and the Endoplasmic Reticulum". N Engl J Med 360 (7): 726-727. http://content.nejm.org/cgi/content/full/360/7/726.
35. ^ Cosnes J (2004). "Tobacco and IBD: relevance in the understanding of disease mechanisms and clinical practice". Best Pract Res Clin Gastroenterol 18 (3): 481–96. doi:10.1016/j.bpg.2003.12.003. PMID 15157822.
36. ^ Lesko S, Kaufman D, Rosenberg L, et al. (1985). "Evidence for an increased risk of Crohn's disease in oral contraceptive users". Gastroenterology 89 (5): 1046–9. PMID 4043662.
37. ^ "Washington Post: Roche Found Liable". http://www.washingtonpost.com/wp-dyn/content/article/2007/05/29/AR2007052901946.html. Retrieved on 2008-06-21.
38. ^ Reddy D, Siegel CA, Sands BE, Kane S (2006). "Possible association between isotretinoin and inflammatory bowel disease". Am. J. Gastroenterol. 101 (7): 1569–73. doi:10.1111/j.1572-0241.2006.00632.x. PMID 16863562.
39. ^ Borobio E, Arín A, Valcayo A, Iñarrairaegui M, Nantes O, Prieto C (2004). "[Isotretinoin and ulcerous colitis]" (in Spanish; Castilian). An Sist Sanit Navar 27 (2): 241–3. PMID 15381956.
40. ^ Reniers DE, Howard JM (2001). "Isotretinoin-induced inflammatory bowel disease in an adolescent". Ann Pharmacother 35 (10): 1214–6. PMID 11675849. http://www.theannals.com/cgi/pmidlookup?view=long&pmid=11675849.
41. ^ "Jury Awards $10.5 Million Over Accutane". http://online.wsj.com/article/SB120890352155036159.html?mod=dist_smartbrief. Retrieved on 2008-06-21.
42. ^ Cobrin GM, Abreu MT (2005). "Defects in mucosal immunity leading to Crohn's disease". Immunol. Rev. 206: 277–95. doi:10.1111/j.0105-2896.2005.00293.x. PMID 16048555.
43. ^ a b Elson, C. (2007), "Monoclonal Anti–Interleukin 23 Reverses Active Colitis in a T Cell–Mediated Model in Mice", Gastroenterology 132: 2359, doi:10.1053/j.gastro.2007.03.104
44. ^ Prescott NJ, Fisher SA, Franke A, et al (2007). "A nonsynonymous SNP in ATG16L1 predisposes to ileal Crohn's disease and is independent of CARD15 and IBD5". Gastroenterology 132 (5): 1665–71. doi:10.1053/j.gastro.2007.03.034. PMID 17484864.
45. ^ {{cite journal| author= Marks DJ, Segal AW. |title=Innate immunity in inflammatory bowel disease: a disease hypothesis|journal= J Pathol. |volume =214 |issue= 4 |pages=260-6 |year=2008 |pmid= 18161747 |url=http://www.ncbi.nlm.nih.gov/pubmed/18161747?ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum
46. ^ {{cite journal| author= Marks DJ, Harbord MW, MacAllister R, Rahman FZ, Young J, Al-Lazikani B, Lees W, Novelli M, Bloom S, Segal AW |title=Defective acute inflammation in Crohn's disease: a clinical investigation |journal= Lancet |volume =367|issue= 9511 |pages=668-78 |year=2006 |pmid= 16503465 |http://www.ncbi.nlm.nih.gov/pubmed/16503465?ordinalpos=14&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum
47. ^ Dessein R, Chamaillard M, Danese S (2008). "Innate immunity in Crohn's disease: the reverse side of the medal". J Clin Gastroenterol 42 (Supplement 3): S144-7. PMID 18806708. http://www.ncbi.nlm.nih.gov/pubmed/18806708?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DiscoveryPanel.Pubmed_Discovery_RA&linkpos=4&log$=relatedreviews&logdbfrom=pubmed.
48. ^ "Crohns Research Overview Page". http://www.crohns.org/research/index.htm.
49. ^ Sartor, R. (2006). "Mechanisms of Disease: pathogenesis of Crohn's disease and ulcerative colitis". Nature Clinical Practice Gastroenterology & Hepatology (3): 390–407. doi:10.1038 (inactive 2008-06-25).
50. ^ Naser SA, Collins MT (2005). "Debate on the lack of evidence of Mycobacterium avium subsp. paratuberculosis in Crohn's disease". Inflamm. Bowel Dis. 11 (12): 1123. PMID 16306778.
51. ^ Giaffer MH, Clark A, Holdsworth CD (1992). "Antibodies to Saccharomyces cerevisiae in patients with Crohn's disease and their possible pathogenic importance". Gut 33 (8): 1071–5. doi:10.1136/gut.33.8.1071. PMID 1398231.
52. ^ Cite error: Invalid tag; no text was provided for refs named scah_out38
53. ^ "Paratuberculosis And Crohn's Disease: Got Milk?". http://www.mad-cow.org/00/paraTB.html.
54. ^ "Two-year outcomes analysis of Crohn's Disease treated with rifabutin and macrolide antibiotics.". http://alan.kennedy.name/crohns/research/chemo/htfull.htm.
55. ^ Baumgart, M., et al. (2007). "Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum (advance online publication)". The ISME Journal 1: 403. doi:10.1038/ismej.2007.52. http://search.nature.com/search/?sp-a=sp1001702d&sp-sfvl-field=subject. – Scholar search
56. ^ "Scientists find how bacteria in cows milk may cause Crohn's disease". University of Liverpool. http://liv.ac.uk/news/press_releases/2007/12/crohns-disease.htm.
57. ^ Cenac N, Andrews CN, Holzhausen M, et al (March 2007). "Role for protease activity in visceral pain in irritable bowel syndrome". J. Clin. Invest. 117 (3): 636–47. doi:10.1172/JCI29255. PMID 17304351.
58. ^ Cenac N, Coelho AM, Nguyen C, et al (November 2002). "Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2". Am. J. Pathol. 161 (5): 1903–15. PMID 12414536. http://ajp.amjpathol.org/cgi/pmidlookup?view=long&pmid=12414536.
59. ^ Boorom KF, Smith H, Nimri L, et al (October 2008). "Oh my aching gut: irritable bowel syndrome, Blastocystis, and asymptomatic infection". Parasit Vectors 1 (1): 40. doi:10.1186/1756-3305-1-40. PMID 18937874.
60. ^ Hugot, Jean-Pierre; Alberti, Corinne; Berrebi, Dominique; Bingen, Edouard; Cézard, Jean-Pierre (2003-12-13), "Crohn's disease: the cold chain hypothesis", The Lancet 362 (9400): 2012-2015, doi:10.1016/S0140-6736(03)15024-6, http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(03)15024-6
61. ^ "Fridges blamed for Crohn's disease rise", Medical News TODAY, 2003-12-12, http://www.medicalnewstoday.com/articles/4849.php
62. ^ Forbes, Alastair; Kalantzis, Tommy (2006), "Crohn's disease : the cold chain hypothesis", International journal of colorectal disease (Berlin: Springer) 21 (5): 399-401, ISSN 0179-1958, http://cat.inist.fr/?aModele=afficheN&cpsidt=17825639
63. ^ Crawford JM. "The Gastrointestinal tract, Chapter 17". In Cotran RS, Kumar V, Robbins SL. Robbins Pathologic Basis of Disease: 5th Edition. W.B. Saunders and Company, Philadelphia, 1994.
64. ^ HCP: Pill Cam, Capsule Endoscopy, Esophageal Endoscopy
65. ^ a b Hara, Amy K.; Jonathan A. Leighton, Russell I. Heigh, Virender K. Sharma, Alvin C. Silva, Giovanni De Petris, Joseph G. Hentz and David E. Fleischer (2006). "Crohn disease of the small bowel: preliminary comparison among CT enterography, capsule endoscopy, small-bowel follow-through, and ileoscopy". Radiology 238 (1): 128–34. doi:10.1148/radiol.2381050296. PMID 16373764.
66. ^ Triester, Stuart L.; Jonathan A. Leighton, Grigoris I. Leontiadis, Suryakanth R. Gurudu, David E. Fleische, Amy K. Hara, Russell I. Heigh, Arthur D. Shiff, and Virender K. Sharma (2006). "A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn's disease. This cannot and should not be used if a stricture or blockage is known, pictures should be taken prior to using the camera since it can get stuck and surgery in the only way to remove it". The American Journal of Gastroenterology 101 (5): 954–64. doi:10.1111/j.1572-0241.2006.00506.x. PMID 16696781.
67. ^ Dixon, P.M.; M.E. Roulston and D.J. Nolan (1993). "The small bowel enema: a ten year review". Clinical Radiology 47 (1): 46–8. doi:10.1016/S0009-9260(05)81213-9. PMID 8428417.
68. ^ Carucci, L. R.; M. S. Levine (2002). "Radiographic imaging of inflammatory bowel disease". Gastroenterology Clinics of North America 31 (1): 93–117. doi:10.1016/S0889-8553(01)00007-3. PMID 12122746.
69. ^ Rajesh, A.; D.D.T. Maglinte (2006). "Multislice CT enteroclysis: technique and clinical applications". Clinical Radiology 61 (1): 31–9. doi:10.1016/j.crad.2005.08.006. PMID 16356814.
70. ^ Zissin, Rivka; Marjorie Hertz, Alexandra Osadchy, Ben Novis and Gabriela Gayer (2005). "Computed Tomographic Findings of Abdominal Complications of Crohn’s Disease—Pictorial Essay" (PDF). Canadian Association of Radiologists Journal 56 (1): 25–35. PMID 15835588. http://www.carj.ca/issues/2005-Feb/25/pg25.pdf. Retrieved on 2006-07-02.
71. ^ MacKalski, B. A.; C. N. Bernstein (2005). "New diagnostic imaging tools for inflammatory bowel disease". Gut 55 (5): 733–41. doi:10.1136/gut.2005.076612. PMID 16609136.
72. ^ Goh, Jason; C. A. O'Morain (2003). "Review article: nutrition and adult inflammatory bowel disease". Alimentary Pharmacology & Therapeutics 17 (3): 307–20. doi:10.1046/j.1365-2036.2003.01482.x. PMID 12562443.
73. ^ Chamouard, Patrick; Zoe Richert, Nicolas Meyer, Gabriel Rahmi, René Baumann (2006). "Diagnostic Value of C-Reactive Protein for Predicting Activity Level of Crohn's Disease". Clinical Gastroenterology and Hepatology 4: 882. doi:10.1016/j.cgh.2006.02.003. PMID 16630759. Epub ahead of print
74. ^ Kaila, B.; K. Orr and C. N. Bernstein (2005). "The anti-Saccharomyces cerevisiae antibody assay in a province-wide practice: accurate in identifying cases of Crohn's disease and predicting inflammatory disease". The Canadian Journal of Gastroenterology 19 (12): 717–21. PMID 16341311. http://www.pulsus.com/Gastro/19_12/kail_ed.htm. Retrieved on 2006-07-02.
75. ^ Israeli, E.; I. Grotto, B. Gilburd, R. D. Balicer, E. Goldin, A. Wiik and Y. Shoenfeld (2005). "Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease". Gut 54 (9): 1232–6. doi:10.1136/gut.2004.060228. PMID 16099791.
76. ^ Faecal calprotectin: a bright future for assessing disease activity in Crohn's disease. Gaya et al. Q J Med 2002; 95: 557-558
77. ^ a b c d Kornbluth, Asher; David B. Sachar (2004). "Ulcerative Colitis Practice Guidelines in Adults" (PDF). American Journal of Gastroenterology 99 (7): 1371–85. doi:10.1111/j.1572-0241.2004.40036.x. PMID 15233681. http://www.acg.gi.org/physicians/guidelines/UlcerativeColitisUpdate.pdf. Retrieved on 2006-11-08.
78. ^ a b c d e Hanauer, Stephen B.; William Sandborn (2001-03-01). "Management of Crohn's Disease in Adults" (PDF). American Journal of Gastroenterology 96 (3): 635–43. doi:10.1111/j.1572-0241.2001.03671.x. PMID 11280528. http://www.acg.gi.org/physicians/guidelines/CrohnsDiseaseinAdults.pdf. Retrieved on 2006-11-08.
79. ^ Broomé, Ulrika; Annika Bergquist (2006). "Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer". Seminars in Liver Disease 26 (1): 31–41. doi:10.1055/s-2006-933561. PMID 16496231.
80. ^ "Clinical Research Alliance Update" (pdf). Crohn's and Colitis Foundation of America. 2007-05-01. http://www.ccfa.org/ccfaprof/research-grant-opps/documents/May-2007-Newsletter.pdf. Retrieved on 2008-02-14.
81. ^ a b Fries, WS (2007-05-16). "Crohn's Disease: 54 Tips to Help You Manage". WebMD. http://www.webmd.com/digestive-disorders/features/crohns-disease-54-tips-to-help-you-manage?ecd=wnl_gid_120607. Retrieved on 2008-02-14.
82. ^ Sandborn, W.J.; Colombel, J.F.; Enns, R.; Feagan, B.G.; Hanauer, S.B.; Lawrance, I.C.; Panaccione, R.; Sanders, M.; Schreiber, S.; Targan, S.; Others, (2005). "Natalizumab Induction and Maintenance Therapy for Crohn's Disease". New England Journal of Medicine 353 (18): 1912. doi:10.1056/NEJMoa043335. PMID 16267322.
83. ^ MacDonald, J.K.; McDonald, J.W.D. (2006). "Natalizumab for induction of remission in Crohn's disease (Cochrane Review)". The Cochrane Database of Systematic Reviews: 1465–858. doi:10.1002/14651858.CD006097. http://dellboy.update-software.com/abstracts/AB006097.htm. Retrieved on 2008-02-15.
84. ^ Longmore, Murray; Ian Wilkinson, Tom Turmezei, Chee Kay Cheung (2007). Oxford Handbook of Clinicial Medicine, 7th edition. Oxford University Press. pp. 266–7. ISBN 0-19-856837-1.
85. ^ Tresca, AJ (2007-01-12). "Resection Surgery for Crohn's Disease". About.com. http://ibdcrohns.about.com/od/surgeryprocedures/a/resectioncrohns.htm. Retrieved on 2008-02-14.
86. ^ Ozuner G, Fazio VW, Lavery IC, Milsom JW, Strong SA (1996). "Reoperative rates for Crohn's disease following strictureplasty. Long-term analysis". Dis. Colon Rectum 39 (11): 1199–203. doi:10.1007/BF02055108. PMID 8918424.
87. ^ Short Bowel Syndrome as defined by the National Institute of Diabetes and Digestive and Kidney Diseases
88. ^ Segal AW et al (2006). "Defective acute inflammation in Crohn's disease: a clinical investigation". Lancet 367 (9511): 668–78. doi:10.1016/S0140-6736(06)68265-2.
89. ^ Croese J, O'neil J, Masson J, et al (2006). "A proof of concept study establishing Necator americanus in Crohn's patients and reservoir donors". Gut 55 (1): 136–7. doi:10.1136/gut.2005.079129. PMID 16344586.
90. ^ Smith J, Stock H, Bingaman S et al (2007). "Low-Dose Naltrexone Therapy Improves Active Crohn’s Disease". American Journal of Gastroenterology 102 (4): 820–8. doi:10.1111/j.1572-0241.2007.01045.x. PMID 17222320.
91. ^ B. Omera, S. Krebsb, H. Omerc and T.O. Noord (2007). "Steroid-sparing effect of wormwood (Artemisia absinthium) in Crohn's disease: A double-blind placebo-controlled study". Phytomedicine 14 (2-3): 87–95. doi:10.1016/j.phymed.2007.01.001.
92. ^ "Bacterium 'to blame for Crohn's'". news.bbc.co.uk. http://news.bbc.co.uk/2/hi/health/7679347.stm. Retrieved on Nov. 23,2008.
93. ^ Canavan, C.; K. R. Abrams, J. Mayberry (2006). "Meta-analysis : colorectal and small bowel cancer risk in patients with Crohn's disease". Alimentary pharmacology & therapeutics 23 (8): 1097–104. doi:10.1111/j.1365-2036.2006.02854.x. ISSN 0269-2813. http://cat.inist.fr/?aModele=afficheN&cpsidt=17660183. Retrieved on 2007-05-23.
94. ^ a b Hiatt, Robert A.; Leon Kaufman (1988). "Epidemiology of inflammatory bowel disease in a defined northern California population". Western Journal of Medicine 149 (5): 541–6. PMID 3250100.
95. ^ Moum, B.; M. H. Vatn, A. Ekbom, E. Aadland, O. Fausa, I. Lygren, N. Stray, J. Sauar, T. Schulz (1996). "Incidence of Crohn's disease in four counties in southeastern Norway, 1990-93. A prospective population-based study. The Inflammatory Bowel South-Eastern Norway (IBSEN) Study Group of Gastroenterologists". Scandinavian Journal of Gastroenterology 31 (4): 355–61. doi:10.3109/00365529609006410. PMID 8726303.
96. ^ Shivananda, S.; J. Lennard-Jones, R. Logan, N. Fear, A. Price, L. Carpenter and M. van Blankenstein (1996). "Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD)". Gut 39 (5): 690–7. doi:10.1136/gut.39.5.690. PMID 9014768.
97. ^ "Crohn's disease manifests differently in boys and girls". CCFA.org. http://www.ccfa.org/reuters/ibdboysgirls.
98. ^ "Who is affected by Crohn's disease". WebMD.com. http://www.webmd.com/hw-popup/who-is-affected-by-crohns-disease.
99. ^ Satsangi J, Jewell DP, Bell JI (1997). "The genetics of inflammatory bowel disease". Gut 40 (5): 572–4. PMID 9203931. PMC: 1027155. http://gut.bmj.com/cgi/pmidlookup?view=long&pmid=9203931.
100. ^ Tysk C, Lindberg E, Järnerot G, Flodérus-Myrhed B (1988). "Ulcerative colitis and Crohn's disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking". Gut 29 (7): 990–6. PMID 3396969. PMC: 1433769. http://gut.bmj.com/cgi/pmidlookup?view=long&pmid=3396969.
101. ^ Kirsner JB (1988). "Historical aspects of inflammatory bowel disease". J. Clin. Gastroenterol. 10 (3): 286–97. PMID 2980764.
الاشتراك في:
الرسائل (Atom)