Alkalosis
Alkalosis refers to a condition reducing hydrogen ion concentration of arterial blood plasma (alkalemia). Generally alkalosis is said to occur when pH of the blood exceeds 7.45. The opposite condition is acidosis.
Types
More specifically, alkalosis can refer to:
• Respiratory alkalosis
• Metabolic alkalosis
Causes
The main cause of respiratory alkalosis is hyperventilation, resulting in a loss of carbon dioxide. Compensatory mechanisms for this would include increased dissociation of the carbonic acid buffering intermediate into hydrogen ions, and the related consumption of bicarbonate,[citation needed] both of which would lower blood pH.
Metabolic alkalosis can be caused by prolonged vomiting, resulting in a loss of hydrochloric acid with the stomach content. Severe dehydration, and the consumption of alkali are other causes. It can also be caused by administration of diuretics and endocrine disorders such as Cushing's syndrome. Compensatory mechanism for metabolic alkalosis involve slowed breathing by the lungs to increase serum carbon dioxide, a condition leaning toward respiratory acidosis. As respiratory acidosis often accompanies the compensation for metabolic alkalosis, and vice versa, a delicate balance is created between these two conditions.
Complications
Metabolic alkalosis is usually accompanied with hypokalemia, causing e.g. muscular weakness, myalgia, and muscle cramps (owing to disturbed function of the skeletal muscles), and constipation (from disturbed function of smooth muscles).
It may also cause hypocalcemia. As the pH of blood increases, the protein in the blood becomes more ionised into anions. This causes the free calcium present in blood to bind strongly with protein. If severe, it may cause tetany (alkalotic tetany).
الثلاثاء، 19 مايو 2009
الأربعاء، 1 أبريل 2009
Graves Disease
Overview of Graves' Disease
Graves’ disease as it is known in the United States and von Basedow’s disease or Parry’s disease in other countries belongs to the organ-specific autoimmune diseases category and is an example of a type II hypersensitivity reaction (McGregor et al., 1998). Graves’ disease is classified by the production of auto-antibodies that bind to the thyroid-stimulating hormone (TSHR) receptor, which is located on the thyroid gland.This action causes an overproduction of thyroid hormones, which is called hyperthyroidism. Graves’ disease is the most common autoimmune disorder that causes hyperthyroidism (Wikipedia contributors et al., 2006).
In Graves’ disease the feedback regulation of thyroid hormone production is disrupted. Normally, the pituitary gland secretes thyroid-stimulating hormones (TSH), which acts on the thyroid inducing the release of thyroid hormones. Feedback suppression occurs when the thyroid hormones act on the pituitary gland suppressing further development of thyroid hormones. Autoimmune B cells that are activated due to autoimmunity secrete auto-antibodies that bind to the TSH receptor located on the thyroid follicle, which also induces thyroid hormone production. The thyroid hormone continues to do its normal job as well as suppressing further development of thyroid hormones by acting on the pituitary gland but has no effect on autoantibody production, which continues to stimulate extreme thyroid hormone production
Figure 1. Is an illustration of excess thyroid hormone production by the thyroid follicle due to auto-antibody production (Janeway et al., 2005).
--------------------------------------------------------------------------------
Symptoms of Graves' Disease
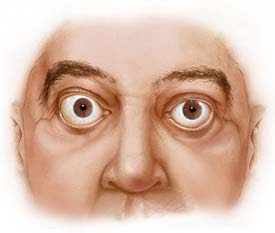
The most common symptoms of Graves’ disease include; diffuse goiter (enlarged thyroid gland), weight loss, rapid heart beat, fatigue, muscle weakness, frequent bowel activity, restlessness, heat sensitivity, hand tremors and increased sweating (National Women's Health Information Center et al., 2006). In addition, certain individuals with Graves' disease display symptoms associated to this form of hyperthyroidism, including infiltrative ophthalmopathy (Figure 2. bulging eyes) and pretibial myxedema (swelling of shins), which occur at lower extremes
Figure 2. This is what a patient's eyes would like if they were infected with Graves' opthalmopathy. Figure taken pending permission from http://www.mayoclinic.org/graves-disease/symptoms.html
--------------------------------------------------------------------------------
Risk Factors
Susceptibility to Graves' disease can be determined by several factors, although the process of initiation is still a mystery. Graves’ disease can occur in genetically susceptible individuals when an environmental factor, possibly an infectious agent damages thyroid tissue leading to the exposure of self-antigens to the immune system. It also has been determined that throughout Graves’ disease appearance it is most common in middle-aged women. There is an 8:1 ratio compared to men. However, it is not scarce in adolescent years, the period of pregnancy or in people over the age of fifty (National Women's Health Information Center et al., 2006).
Different factors of evidence have proposed that Graves' disease occurs in families. Horwitz and Refetoff et al. (1977) found and proprosed that Graves' disease has an increased occurrence in patients with familial deficiency of thyroxine-binding globulin.
Unfortunately, Graves’ disease can be passed through pregnancy. Mothers with this disease transfer their auto-antibodies which are IgG mediated across the placenta into the fetus. The newborn develops slight symptoms of Graves’ disease. Therefore, newborns are said to be born with hyperthyroidism. This transfer of antibody-mediated autoimmune disease can be fixed by a process called plasmapheresis, which replaces the newborn’s plasma with normal plasma, thus eliminating the transferred maternal antibody (Janeway et al., 2005).
Organ-specific autoimmune diseases are considered to occur frequently together in many combinations; for example, autoimmune thyroid disease and vitiligo (autoimmune depigmenting disease) are occasionally found in the same individual. In addition, these diseases can also be recognized with associations of Human Leukocyte Antigen (HLA) serotype. (Janeway et al., 2005). Association of HLA serotype with susceptibility to Graves’ disease is with the HLA-DQ gene, which is in linkage to the DR genes. The HLA allele associated with Graves’ disease is said to be allele DR3 (McGregor et al., 1998). We do not want to overlook genetic factors but they are not determinative for predisposition to Graves' disease.
Genetic analysis has shown that Graves’ disease is associated with the polymorphisms in the cytotoxic T-lymphocyte antigen 4 (CTLA-4) genes (DeGroot et al., 2005). Allele sequence of A/G or G/G on nucleotide 49 of the CTLA-4 gene in patients have shown that more people are infected with Graves' Disease (Kouki et al., 2000).
--------------------------------------------------------------------------------
Immune System and Graves' Disease
The thyroid gland is vital in maintaining the body’s homeostasis through the secretion of the thyroid hormones, thyroxine (T4) and triiodothyronine (T3). These thyroid hormones are secreted by thyrocytes through fluid phase uptake and lysosomal degradation of the prohormone thyroglobulin (Tg). Hyperthyroidism occurs when there is an overproduction of these thyroid hormones.
The body has a negative-feedback pathway that controls hyperthyroidism (refer back to Figure 1). Immune system signaling is important for hormone synthesis to be controlled. Consider immune system signaling in assisting TSH's up-regulation of hormone synthesis and in turn TSH's down-regulation by thyroid hormones themselves. This cycle is what helps stablize hyperthyroidism from occurring in less susceptible individuals (Lisi et al., 2003).
Graves’ disease can occur in genetically susceptible individuals when an environmental factor, possibly an infectious agent damages the thyroid leading to the exposure of self-antigens to the immune system. Infectious agents have been considered an important environmental inducer of autoimmune diseases. For example, Yersinia Enterocolitica is a common intestinal parasite, which may induce reactivity to TSH receptor. Within the pathogenic bacteria, the TSH receptor could actually appear to be coded for by the bacteria’s plasmids. This concept can be referred as molecular mimicry. A substantial amount of individuals who are affected by Graves’ disease have been infected with this bacteria compared to people who do not have Graves' disease. If this bacterium is exposed to your body as well as your immune system, it can induce TSH receptor antibodies and TSH would appear to bind to a molecule on the bacteria leading to an overproduction of thyroid-stimulating hormones (DeGroot et al., 2005).
Certain cells such as lymphocytes and thyroid can further increase autoimmune responses in Graves' disease. Thyroid cells are able to interact with the immune system causing a response where the symptoms of hyperthyroidism worsen. Unfortunately, thyrocytes that make up the thyroid follicle express and synthesize certain proteins that they normally would not (Weetman et al., 2000). When there is an over-production of thyroid hormones thyroid-reactive T cells are formed and they infiltrate the thyroid gland. In Graves' disease, the majority of the T cells differentiate into Th2 instead of Th1 cells where they can activate B cells which produce TSHR antibodies. This differentiation into Th2 cells allows the stimulation of the thyroid, which causes clinical hyperthyroidism (Davies et al., 2000). In contrast, Hashimoto thyroiditis occurs in the opposite direction as Graves' disease by infilrating T cells differentiating into Th1 cells, which thyroid follicular cells undergo apoptosis leading to clinical hypothyroidism (underactivity/failure of the thyroid) (Weetman et al., 1996).
--------------------------------------------------------------------------------
Current Treatments of Graves' Disease
People who develop this autoimmune disease try to treat it with one of the three different type of treatments such as anti-thyroid drugs, radioactive iodine or surgical thyroid removal. Radioactive idoine is the most common treatment for Graves' disease in North America.
Anti-thyroid drugs can decrease the production of excessive thyroid hormones produced by the thyroid, sustaining thyroid hormone synthesis back to its threshold. Radioactive iodine decreases thyroid hormone levels by damaging thyroid cells, which eventually shrinks the cells leading to their destruction and as well as the functioning of the thyroid gland. Surgical treatment is the removal of the entire thyroid gland, which in many cases people develop an under-active thyroid. If an individual decides to take the surgical route they will have to take thyroid replacement hormones for the duration of their life (National Women's Health Information Center et al., 2006).
The main anti-thyroid drug used in the United States is called methimazole (Wikipedia contributors et al., 2006). “Methimazole…restores euthyroidism by inhibiting thyroperoxidase activity and hormone formation within Tg" (Lisi et al., 2003).
Graves’ disease as it is known in the United States and von Basedow’s disease or Parry’s disease in other countries belongs to the organ-specific autoimmune diseases category and is an example of a type II hypersensitivity reaction (McGregor et al., 1998). Graves’ disease is classified by the production of auto-antibodies that bind to the thyroid-stimulating hormone (TSHR) receptor, which is located on the thyroid gland.This action causes an overproduction of thyroid hormones, which is called hyperthyroidism. Graves’ disease is the most common autoimmune disorder that causes hyperthyroidism (Wikipedia contributors et al., 2006).
In Graves’ disease the feedback regulation of thyroid hormone production is disrupted. Normally, the pituitary gland secretes thyroid-stimulating hormones (TSH), which acts on the thyroid inducing the release of thyroid hormones. Feedback suppression occurs when the thyroid hormones act on the pituitary gland suppressing further development of thyroid hormones. Autoimmune B cells that are activated due to autoimmunity secrete auto-antibodies that bind to the TSH receptor located on the thyroid follicle, which also induces thyroid hormone production. The thyroid hormone continues to do its normal job as well as suppressing further development of thyroid hormones by acting on the pituitary gland but has no effect on autoantibody production, which continues to stimulate extreme thyroid hormone production
Figure 1. Is an illustration of excess thyroid hormone production by the thyroid follicle due to auto-antibody production (Janeway et al., 2005).
--------------------------------------------------------------------------------
Symptoms of Graves' Disease
The most common symptoms of Graves’ disease include; diffuse goiter (enlarged thyroid gland), weight loss, rapid heart beat, fatigue, muscle weakness, frequent bowel activity, restlessness, heat sensitivity, hand tremors and increased sweating (National Women's Health Information Center et al., 2006). In addition, certain individuals with Graves' disease display symptoms associated to this form of hyperthyroidism, including infiltrative ophthalmopathy (Figure 2. bulging eyes) and pretibial myxedema (swelling of shins), which occur at lower extremes
Figure 2. This is what a patient's eyes would like if they were infected with Graves' opthalmopathy. Figure taken pending permission from http://www.mayoclinic.org/graves-disease/symptoms.html
--------------------------------------------------------------------------------
Risk Factors
Susceptibility to Graves' disease can be determined by several factors, although the process of initiation is still a mystery. Graves’ disease can occur in genetically susceptible individuals when an environmental factor, possibly an infectious agent damages thyroid tissue leading to the exposure of self-antigens to the immune system. It also has been determined that throughout Graves’ disease appearance it is most common in middle-aged women. There is an 8:1 ratio compared to men. However, it is not scarce in adolescent years, the period of pregnancy or in people over the age of fifty (National Women's Health Information Center et al., 2006).
Different factors of evidence have proposed that Graves' disease occurs in families. Horwitz and Refetoff et al. (1977) found and proprosed that Graves' disease has an increased occurrence in patients with familial deficiency of thyroxine-binding globulin.
Unfortunately, Graves’ disease can be passed through pregnancy. Mothers with this disease transfer their auto-antibodies which are IgG mediated across the placenta into the fetus. The newborn develops slight symptoms of Graves’ disease. Therefore, newborns are said to be born with hyperthyroidism. This transfer of antibody-mediated autoimmune disease can be fixed by a process called plasmapheresis, which replaces the newborn’s plasma with normal plasma, thus eliminating the transferred maternal antibody (Janeway et al., 2005).
Organ-specific autoimmune diseases are considered to occur frequently together in many combinations; for example, autoimmune thyroid disease and vitiligo (autoimmune depigmenting disease) are occasionally found in the same individual. In addition, these diseases can also be recognized with associations of Human Leukocyte Antigen (HLA) serotype. (Janeway et al., 2005). Association of HLA serotype with susceptibility to Graves’ disease is with the HLA-DQ gene, which is in linkage to the DR genes. The HLA allele associated with Graves’ disease is said to be allele DR3 (McGregor et al., 1998). We do not want to overlook genetic factors but they are not determinative for predisposition to Graves' disease.
Genetic analysis has shown that Graves’ disease is associated with the polymorphisms in the cytotoxic T-lymphocyte antigen 4 (CTLA-4) genes (DeGroot et al., 2005). Allele sequence of A/G or G/G on nucleotide 49 of the CTLA-4 gene in patients have shown that more people are infected with Graves' Disease (Kouki et al., 2000).
--------------------------------------------------------------------------------
Immune System and Graves' Disease
The thyroid gland is vital in maintaining the body’s homeostasis through the secretion of the thyroid hormones, thyroxine (T4) and triiodothyronine (T3). These thyroid hormones are secreted by thyrocytes through fluid phase uptake and lysosomal degradation of the prohormone thyroglobulin (Tg). Hyperthyroidism occurs when there is an overproduction of these thyroid hormones.
The body has a negative-feedback pathway that controls hyperthyroidism (refer back to Figure 1). Immune system signaling is important for hormone synthesis to be controlled. Consider immune system signaling in assisting TSH's up-regulation of hormone synthesis and in turn TSH's down-regulation by thyroid hormones themselves. This cycle is what helps stablize hyperthyroidism from occurring in less susceptible individuals (Lisi et al., 2003).
Graves’ disease can occur in genetically susceptible individuals when an environmental factor, possibly an infectious agent damages the thyroid leading to the exposure of self-antigens to the immune system. Infectious agents have been considered an important environmental inducer of autoimmune diseases. For example, Yersinia Enterocolitica is a common intestinal parasite, which may induce reactivity to TSH receptor. Within the pathogenic bacteria, the TSH receptor could actually appear to be coded for by the bacteria’s plasmids. This concept can be referred as molecular mimicry. A substantial amount of individuals who are affected by Graves’ disease have been infected with this bacteria compared to people who do not have Graves' disease. If this bacterium is exposed to your body as well as your immune system, it can induce TSH receptor antibodies and TSH would appear to bind to a molecule on the bacteria leading to an overproduction of thyroid-stimulating hormones (DeGroot et al., 2005).
Certain cells such as lymphocytes and thyroid can further increase autoimmune responses in Graves' disease. Thyroid cells are able to interact with the immune system causing a response where the symptoms of hyperthyroidism worsen. Unfortunately, thyrocytes that make up the thyroid follicle express and synthesize certain proteins that they normally would not (Weetman et al., 2000). When there is an over-production of thyroid hormones thyroid-reactive T cells are formed and they infiltrate the thyroid gland. In Graves' disease, the majority of the T cells differentiate into Th2 instead of Th1 cells where they can activate B cells which produce TSHR antibodies. This differentiation into Th2 cells allows the stimulation of the thyroid, which causes clinical hyperthyroidism (Davies et al., 2000). In contrast, Hashimoto thyroiditis occurs in the opposite direction as Graves' disease by infilrating T cells differentiating into Th1 cells, which thyroid follicular cells undergo apoptosis leading to clinical hypothyroidism (underactivity/failure of the thyroid) (Weetman et al., 1996).
--------------------------------------------------------------------------------
Current Treatments of Graves' Disease
People who develop this autoimmune disease try to treat it with one of the three different type of treatments such as anti-thyroid drugs, radioactive iodine or surgical thyroid removal. Radioactive idoine is the most common treatment for Graves' disease in North America.
Anti-thyroid drugs can decrease the production of excessive thyroid hormones produced by the thyroid, sustaining thyroid hormone synthesis back to its threshold. Radioactive iodine decreases thyroid hormone levels by damaging thyroid cells, which eventually shrinks the cells leading to their destruction and as well as the functioning of the thyroid gland. Surgical treatment is the removal of the entire thyroid gland, which in many cases people develop an under-active thyroid. If an individual decides to take the surgical route they will have to take thyroid replacement hormones for the duration of their life (National Women's Health Information Center et al., 2006).
The main anti-thyroid drug used in the United States is called methimazole (Wikipedia contributors et al., 2006). “Methimazole…restores euthyroidism by inhibiting thyroperoxidase activity and hormone formation within Tg" (Lisi et al., 2003).
الثلاثاء، 31 مارس 2009
Cardiac catheterization
Radiocontrast in the left ventricle during cardiac catheterization (ventriculogram).
Visualization of the coronary arteries (in this case, the left coronary artery which gives the circumflex branch of left coronary artery).
Cardiac catheterization (heart cath) is the insertion of a catheter into a chamber or vessel of the heart. This is done for both investigational and interventional purposes. Coronary catheterization is a subset of this technique, involving the catheterization of the coronary arteries.
A small puncture is made in a vessel in the groin, the inner bend of the elbow, or neck area (the femoral vessels or the carotid/jugular vessels), then a guidewire is inserted into the incision and threaded through the vessel into the area of the heart that requires treatment, visualized by fluoroscopy or echocardiogram, and a catheter is then threaded over the guidewire. If X-ray fluoroscopy is used, a radiocontrast agent will be administered to the patient during the procedure. When the necessary procedures are complete, the catheter is removed. Firm pressure is applied to the site to prevent bleeding. This may be done by hand or with a mechanical device. Other closure techniques include an internal suture. If the femoral artery was used, the patient will probably be asked to lie flat for several hours to prevent bleeding or the development of a hematoma. Cardiac interventions such as the insertion of a stent prolong both the procedure itself as well as the post-catheterization time spent in allowing the wound to clot.
A cardiac catheterization is a general term for a group of procedures that are performed using this method, such as coronary angiography, as well as left ventrical angiography. Once the catheter is in place, it can be used to perform a number of procedures including angioplasty, angiography, and balloon septostomy.
History
Main article: History of invasive and interventional cardiology
The history of cardiac catheterization dates back to Claude Bernard (1813-1878), who used it on animal models. Clinical application of cardiac catheterization begins with Werner Forssmann in the 1930s, who inserted a catheter into the vein of his own forearm, guided it fluoroscopically into his right atrium, and took an X-ray picture of it. Forssmann won the Nobel Prize in Physiology or Medicine for this achievement. During World War II, André Frédéric Cournand and his colleagues developed techniques for left and right heart catheterization. Indications for investigational use
This technique has several goals:
• confirm the presence of a suspected heart ailment
• quantify the severity of the disease and its effect on the heart
• seek out the cause of a symptom such as shortness of breath or signs of cardiac insufficiency
• make a patient assessment prior to heart surgery
Investigative techniques used with cardiac catheterization
A probe that is opaque to X-rays is inserted into the left or right chambers of the heart for the following reasons:
• to measure intracardiac and intravascular blood pressures
• to take tissue samples for biopsy
• to inject various agents for measuring blood flow in the heart; also to detect and quantify the presence of an intracardiac shunt
• to inject contrast agents in order to study the shape of the heart vessels and chambers and how they change as the heart beats
Radiocontrast in the left ventricle during cardiac catheterization (ventriculogram).
Visualization of the coronary arteries (in this case, the left coronary artery which gives the circumflex branch of left coronary artery).
Cardiac catheterization (heart cath) is the insertion of a catheter into a chamber or vessel of the heart. This is done for both investigational and interventional purposes. Coronary catheterization is a subset of this technique, involving the catheterization of the coronary arteries.
A small puncture is made in a vessel in the groin, the inner bend of the elbow, or neck area (the femoral vessels or the carotid/jugular vessels), then a guidewire is inserted into the incision and threaded through the vessel into the area of the heart that requires treatment, visualized by fluoroscopy or echocardiogram, and a catheter is then threaded over the guidewire. If X-ray fluoroscopy is used, a radiocontrast agent will be administered to the patient during the procedure. When the necessary procedures are complete, the catheter is removed. Firm pressure is applied to the site to prevent bleeding. This may be done by hand or with a mechanical device. Other closure techniques include an internal suture. If the femoral artery was used, the patient will probably be asked to lie flat for several hours to prevent bleeding or the development of a hematoma. Cardiac interventions such as the insertion of a stent prolong both the procedure itself as well as the post-catheterization time spent in allowing the wound to clot.
A cardiac catheterization is a general term for a group of procedures that are performed using this method, such as coronary angiography, as well as left ventrical angiography. Once the catheter is in place, it can be used to perform a number of procedures including angioplasty, angiography, and balloon septostomy.
History
Main article: History of invasive and interventional cardiology
The history of cardiac catheterization dates back to Claude Bernard (1813-1878), who used it on animal models. Clinical application of cardiac catheterization begins with Werner Forssmann in the 1930s, who inserted a catheter into the vein of his own forearm, guided it fluoroscopically into his right atrium, and took an X-ray picture of it. Forssmann won the Nobel Prize in Physiology or Medicine for this achievement. During World War II, André Frédéric Cournand and his colleagues developed techniques for left and right heart catheterization. Indications for investigational use
This technique has several goals:
• confirm the presence of a suspected heart ailment
• quantify the severity of the disease and its effect on the heart
• seek out the cause of a symptom such as shortness of breath or signs of cardiac insufficiency
• make a patient assessment prior to heart surgery
Investigative techniques used with cardiac catheterization
A probe that is opaque to X-rays is inserted into the left or right chambers of the heart for the following reasons:
• to measure intracardiac and intravascular blood pressures
• to take tissue samples for biopsy
• to inject various agents for measuring blood flow in the heart; also to detect and quantify the presence of an intracardiac shunt
• to inject contrast agents in order to study the shape of the heart vessels and chambers and how they change as the heart beats
الاشتراك في:
الرسائل (Atom)